Scroll to:
Chronic endometritis and infertility — in vitro fertilization outcomes: systematic review and meta-analysis
https://doi.org/10.25207/1608-6228-2023-30-5-15-40
Abstract
Background. The relevance of the problem is related to the high prevalence of chronic endometritis (CE); its role in female infertility, implantation failures during assisted reproductive technology procedures, and recurrent miscarriage; as well as the lack of a unified strategy in the diagnosis and treatment of this pathology. The present systematic review with a meta-analysis focuses on evaluating the impact of CE and its therapy on the outcome of in vitro fertilization. In addition, the effect of CE of various severity on the outcomes of assisted reproductive technologies is analyzed. Objective. To analyze the effect of CE of varying severity and its treatment on the outcomes of in vitro fertilization. Methods. Using PubMed, Medline, Scopus, Embase, ELibrary, Cochrane Central Register of Controlled Trials (CENTRAL), WHO International Clinical Trials Registry, and Russian Science Citation Index, a systematic search was conducted for articles published over the past 12 years that met the following criteria: randomized controlled trial examining the effect of CE of varying severity on fertility and ways to treat it. The following indicators were calculated: ongoing pregnancy/live birth, clinical pregnancy, and miscarriage rates. A total of 4145 patients (from ten studies) were included. A meta-analysis was performed using Stata 11.0 software (The Cochrane Collaboration, Oxford, UK). The heterogeneity was considered low at I2 <30%, moderate at 30–50%, and high at >50%. Results. Women with CE exhibited lower ongoing pregnancy/live birth (OR 1.97; p = 0.02) and clinical pregnancy rates (OR 2.28; p = 0.002) as compared to women without it. CE treatment increased the ongoing pregnancy/live birth (OR 5.33; p < 0.0001) and clinical pregnancy rates (OR 3.64; p = 0.0001). In vitro fertilization outcomes were comparable in women treated for CE and women without CE (ongoing pregnancy/live birth rate, clinical pregnancy rate, and miscarriage rate: p = ns). Women with severe CE exhibited lower ongoing pregnancy/live birth (OR 0.43; p = 0.003) and clinical pregnancy rates (OR 0.40; p = 0.0007). Mild CE showed no significant effect on in vitro fertilization outcomes (ongoing pregnancy/ live birth rate, clinical pregnancy rate, and miscarriage rate: p = ns). Conclusion. The conducted meta-analysis showed that CE significantly reduces the ongoing pregnancy/live birth and clinical pregnancy rates in infertile women undergoing in vitro fertilization. Noteworthy is that antimicrobial therapy in such patients improves the results of assisted reproductive technologies, which are comparable to those of patients without CE. The negative impact of this pathology on the implantation capacity of the endometrium is most often observed in the severe form, while its mild form has virtually no effect on the in vitro fertilization outcome.
Keywords
For citations:
Lokshin V.N., Kutsenko I.I., Borovikov I.O., Bulgakova V.P., Kravtsova E.I., Biryukova M.I., Borovikova O.I., Nikogda J.V. Chronic endometritis and infertility — in vitro fertilization outcomes: systematic review and meta-analysis. Kuban Scientific Medical Bulletin. 2023;30(5):15-40. https://doi.org/10.25207/1608-6228-2023-30-5-15-40
INTRODUCTION
The currently accepted definition of chronic endometritis (CE) was given in 2018 by I. Moreno, E. Cicinelli, et al.: a chronic inflammation of the endometrium caused by its abnormal microbiome [1]. Recent years have seen an increasing interest in this pathology, primarily due to its presumed role in infertility, recurrent miscarriage, and recurrent implantation failure (RIF) during in vitro fertilization (IVF) procedures [2–10]. It is noted that the prevalence of CE exceeds 30% in these conditions [11–14].
A variety of theories are available to explain the CE-related reduction in the implantation capacity of the endometrium [15–20] — activation of local inflammatory processes with altered secretion of cytokines and chemokines [19][21–25]; abnormal leukocytic infiltration, decidualization, and vascularization in the uterine cavity mucosa [13][19][23][24][26][27][29][30]; altered uterine contractility [16][28]. Although these theories clearly reflect some features of CE pathogenesis, the current evidence for a correlation between this pathology and implantation defects is often based on data from studies that are not without their limitations, such as heterogeneous sampling and questionable diagnostic criteria for CE [31][32]. Therefore, the scientific community remains divided between those who are for and against recognizing CE as the real cause of female infertility.
One of the most frequently asked questions regarding CE is the methodology used to diagnose it. Hysteroscopy has sufficient sensitivity; however, it is highly dependent on the technician [33–35]. Therefore, the current gold standard for CE diagnosis is the detection of plasma cells (PCs) in endometrial biopsy material [36–38]. The number of PCs required to diagnose CE remains a matter of discussion [38][39].
In this systematic review involving a meta-analysis, we examined scientific and clinical studies of infertile women with repeated IVF failures; specifically, we studied the role of CE in reducing IVF effectiveness, evaluated the impact of CE treatment on IVF outcomes, and analyzed the propensity to implantation impairment depending on CE severity (determined by plasma cell infiltration).
The article aims to analyze the effect of chronic endometritis of varying severity and its treatment on the outcomes of in vitro fertilization.
METHODS
Study design
The systematic review with meta-analysis was reported in line with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [40].
Eligibility criteria
The evaluation of studies for compliance with the inclusion criteria was conducted in three stages: evaluation of the title, abstract, and full text of the article.
Inclusion criteria
All studies (experimental and observational) evaluating the impact of chronic endometritis on the outcome of in vitro fertilization.
Exclusion criteria
Studies that include patients with recurrent miscarriage; evaluate spontaneous conception rate in women with CE; evaluate other types of endometrial inflammation (acute, subacute, or tuberculous endometritis).
Diagnostic criteria
Chronic endometritis was defined as the presence of at least one endometrial stromal plasma cell in the entire section; immunohistochemical analysis was used as an evaluation method for CD138+ cells (syndecan-1 staining). Severe CE was defined as the presence of ≥5 PCs in the endometrial tissue per HPF, while the mild form was defined as the presence of 1–4 PCs per HPF.
The following comparisons were made: 1) CE patients vs non-CE patients (with normal endometrial histology); 2) patients with CE (untreated or persistent after antibiotic therapy) vs treated CE, i.e., following antibiotic therapy (endometrial biopsy showed no CE); 3) treated CE patients (following antibiotic therapy) vs non-CE patients (with normal endometrial histology).
Also, a comparison was conducted between CE patients, which was defined by the number of PCs in endometrial tissue: ≥5 PCs per HPF (severe CE) vs 1–4 PCs (mild CE). Also, a subgroup of patients with 1–4 PCs was compared with non-CE patients.
Information sources
Two authors conducted an independent search for publications in all languages across electronic databases (PubMed, Medline, Scopus, Embase, ELibrary, Cochrane Central Register of Controlled Trials (CENTRAL), WHO International Clinical Trials Registry, and RSCI) that were published between January 2010 and January 2022.
Search strategy
The search query included the following words: chronic endometritis; infertility; endometrial plasma cells (EPC); endometrial CD-138; hysteroscopy; in vitro fertilization (IVF); assisted reproductive technology (ART); embryo transfer; recurrent implantation failure (RIF). For domestic databases, Russian equivalents for these terms were used.
Study selection
Initially, the title and abstract of potential studies were examined, and in case of insufficient information, the full text of the article was studied. Reviews, case histories, comments, and letters were excluded. In order to increase the transparency of the method, we selected only randomized controlled trials (RCTs) in human beings. The references of all found studies were examined to identify additional, previously undiscovered publications. Duplicate results obtained in the search across different databases were also excluded.
Data collection
Two researchers independently examined databases and found potentially relevant studies. Then, another reviewer assessed whether the full text of the articles met the inclusion criteria. Points at issue were resolved through discussion. For the sake of transparency and complete reporting of the meta-analysis, the search for studies was presented as a PRISMA block diagram.
Data and generalized effect size
With the aim to establish the relationship between chronic endometritis and in vitro fertilization outcomes, the meta-analysis used the following indicators as primary endpoints: ongoing pregnancy or live birth rate (per patient — OPR/LBR), clinical pregnancy rate (per patient — CPR), and miscarriage rate (per clinical pregnancy — MR). OPR/LBR (“ongoing pregnancy”) — after 12 weeks of gestation; “live birth” — birth of one or more viable children; CPR — presence of an intrauterine gestational sac as indicated by transvaginal ultrasound or other reliable clinical signs; MR — fetal loss before 20 weeks of gestation expressed as mean values (M) ± standard deviations (SD).
Data retrieval and quality assessment
RCT data meeting inclusion and exclusion criteria were obtained by the reviewers according to the predefined criteria. Special attention was paid to data concerning severity, diagnostic criteria for chronic endometritis, its therapy methods, and treatment outcomes (biochemical pregnancy, clinical pregnancy, live birth rate, and miscarriage rate). All authors independently assessed the risk of systematic error in each study using guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (www.handbook.cochran.org). In the course of the study, reviewers also evaluated the randomization method; the degree of blinding of patients, medical staff, and researchers, as well as the availability and completeness of data on outcomes presented by the authors.
Statistical analysis
The meta-analysis was performed using Stata 11.0 and Review Manager (version 5.4, The Cochrane Collaboration, 2020, Oxford, UK). The study results were expressed using an odds ratio (OR) with a 95% confidence interval (95% CI); values of p < 0.05 were considered statistically significant.
Synthesis of results
The heterogeneity was considered low at I2 <30%, moderate at 30–50%, and high at >50%. The relation I2>50% indicated significant heterogeneity between studies and prevented reliance on a combination of study results, so a random effects model was used to summarize the effects. The random effects model (DerSimonian & Laird method) was applied in the meta-analysis [41]. The analysis of subgroups and sensitivity was used to examine the sources of heterogeneity in the studies. The condition was to follow the Cochrane Handbook recommendations for assessing publication bias [42].
Risk of bias in individual studies
The recommendations provided in the Cochrane Handbook for Systematic Reviews were used to assess the risk of bias in RCTs. The following criteria were assessed: presence of randomization; allocation concealment; blinding of participants and staff; blinding of the researcher evaluating outcomes; presence of incomplete data on outcomes; selective reporting of data; and other risks of bias. The systematic error risk assessment was denoted as follows: low risk — “+”; questionable risk of bias, as well as incompletely reported data — “?”; high risk of bias — “−.”
Additional analyses
No additional analyses were planned for this study.
RESULTS
Selection of studies
We conducted a search for papers published between January 2010 and January 2022 that met the inclusion criteria. The search scheme is presented in Fig. 1.
Fig. 1. Block diagram of the study design
Note. The block diagram was created by the authors
(as per PRISMA recommendations).
Рис. 1. Блок-схема дизайна проведенного исследования
Примечание: блок-схема выполнена авторами
(согласно рекомендациям PRISMA).
After removing duplicate publications, the search yielded 1905 relevant studies; of these, 19 full-text articles that met the eligibility criteria for this meta-analysis were selected. After evaluating the full text, a total of ten studies in the English language were included in this meta-analysis [6][9][11][12][14][17][18][44][45][46]. The most important data from the ten RCTs included in the analysis are summarized in Table 1.
Table 1. Characteristics of the studies
Таблица 1. Характеристика исследований
|
Authors and year |
Design, period, country |
Participants, main inclusion criteria |
IVF cycle |
Procedure |
Diagnostic criteria for CE |
Groups |
Results |
|
Cicinelli et al. 2015 [6] |
prospective, 2009–2012, Italy |
106 RIF patients undergoing an IVF cycle - unexplained infertility; - age of <40 years |
oocyte retrieval 34 h following ovulation induction; - ≤3 embryos; - transfer at day 3 |
hysteroscopy, biopsy, morphological examination, and endometrial culture - antibiotics - IVF cycle monitoring |
1–5 CD138+ cells or discrete <20 PC clusters |
Group A: treated CE patients (n = 46); Group B: recurrent CE patients (n = 15) |
- clinical pregnancy rate - ongoing pregnancy/live birth rate - miscarriage rate |
|
Demirdag et al. 2021 [17] |
retrospective, 2016–2019, Turkey |
1164 patients undergoing an IVF cycle (232 RIF) - age of <40 years |
- oocyte retrieval 36 h following ovulation induction; - 1–2 embryos; - transfer at days 3–5 |
biopsy and morphological examination - antibiotic therapy - IVF cycle monitoring |
≥1 CD138+ cell per HPF |
Group 1: treated CE patients (n = 129); Group 2: no-CE patients (n = 103); Group 3: first IVF cycle (n = 932) |
- implantation rate - clinical pregnancy rate - live birth rate |
|
Fan et al. 2019 [45] |
retrospective, 2016–2018, China |
141 patients aged 20–38 years undergoing the 1st IVF cycle |
– |
biopsy and morphological examination - IVF cycle monitoring |
≥1 CD138+ cell per HPF or mm² |
Group 1: <1 CD138 cells (n = 97) Group 2: ≥1 CD138 cells (n = 44) |
- implantation rate - clinical pregnancy rate |
|
Hirata et al. 2021 [44] |
prospective, 2014–2017, Japan |
53 patients undergoing an IVF cycle - unexplained infertility; - age of <41 years |
oocyte retrieval and freezing - transfer within 90 days of endometrial biopsy |
hysteroscopy, biopsy, and morphological examination |
four criteria: - ≥2 |
according to the criterion: (≥1; 2; 3, 4) Group A: CE patients (26; 19; 14; 11) Group B: non-CE patients (27; 34; 39; 42) |
- clinical pregnancy rate - live birth rate - miscarriage rate |
|
Johnston-Mac Ananny et al. 2010 [9] |
prospective, 2001–2007, US |
518 RIF patients undergoing an ART cycle: 33 patients with endometrial biopsy and 485 patients without biopsy |
oocyte retrieval 35 h following ovulation induction |
biopsy and morphological examination - antibiotic therapy - IVF cycle monitoring |
≥1 CD138+ cell per HPF |
Group 1: treated CE patients (n = 10); Group 2: non-CE patients (n = 23); Group 3: RIF patients, no endometrial biopsy (n = 485) |
- clinical pregnancy rate - ongoing pregnancy rate |
|
Kitaya et al. 2017 [18] |
prospective cohort, 2011–2014, Japan |
421 RIF patients who underwent up to three IVF cycles |
– |
hysteroscopy, biopsy, and morphological examination - antibiotic therapy - IVF cycle monitoring |
endometrial stromal plasmacyte density index (ESPDI) of ≥0.25 (stromal PCs / total number of PCs) |
Group A: treated CE patients (n = 116); Group B: recurrent CE patients (n = 4); Group C: non-CE patients (n = 226) |
- clinical pregnancy rate - ongoing pregnancy/ live birth rate - miscarriage rate |
|
Kuroda et al. 2020 [11] |
crossover study, 2018–2020, Japan |
88 infertile women |
ovulation stimulation (follicle size of ≥17 mm); - oocyte retrieval 35 h following ovulation induction - IVF and vitrification |
biopsy and morphological examination - ERA - antibiotic therapy - IVF cycle monitoring |
≥5 CD138+ cells per ten random stromal areas |
Group A: non-CE patients (n = 33); Group B: CE patients (n = 19), ERA; Group C: treated CE patients (n = 36) |
- hCG+ - clinical pregnancy rate - miscarriage rate - ongoing pregnancy rate |
|
Li et al. 2021 [12] |
retrospective, 2017–2018, China |
716 infertile patients undergoing an IVF cycle - age of <45 years; - previous antibiotic treatment for CE |
– |
- endometrial scratching - biopsy and morphological examination - IVF cycle monitoring |
six diagnostic criteria - 0–4 PCs per 30 HPF; - ≥5 PCs per 30 HPF |
Group A: 0 PCs (n = 433); Group B: 1 PC (n = 178); Group C: Group D: 3 PCs (n = 18); Group E: Group F: ≥5 PCs (n = 38) |
- clinical pregnancy rate - live birth rate - miscarriage rate |
|
Xiong et al. 2021 [14] |
retrospective, 2017–2018 China |
640 patients undergoing an IVF cycle - without antibiotics prior to hysteroscopy - age of <40 years; - BMI of <30 kg/m² - transfer cycles within six months following antibiotics |
ovulation stimulation at a follicle size of 18 mm; - oocyte retrieval 36 h following hCG administration |
hysteroscopy, biopsy, and morphological examination - antibiotic therapy - IVF cycle monitoring |
≥1 PC per HPF |
Group 1: 0 PCs (n = 88); Group 2: 1–4 PCs with antibiotic treatment (n = 116); Group 3: 1–4 PCs without antibiotic treatment (n = 199). ------- Group 2: treated CE (n = 211); ------- |
- implantation rate - clinical pregnancy rate - live birth rate - early pregnancy loss rate - cumulative live birth rate |
|
Zhang et al. 2019 [46] |
prospective cohort, 2015–2017, China |
298 RIF patients undergoing the 1st IVF cycle - age of <35 years; - ≥3 failed IVF cycles |
ovulation stimulation at a follicle size of 17 mm - oocyte retrieval 36 h following ovulation induction |
hysteroscopy, biopsy, and morphological examination - intrauterine antibiotic therapy - IVF cycle monitoring |
≥1 PC per HPF |
Group 1: non-CE patients (n = 126); Group 2: treated CE patients (n = 85); Group 3: recurrent CE patients (n = 24) |
- implantation rate - clinical pregnancy rate - live birth rate - clinical losses |
Note. The table was compiled by the authors;
Abbreviations:
CD — Cluster of Differentiation;
ERA — Endometrial Receptivity Analysis;
ESPDI — endometrial stromal plasmacyte density index;
RIF — Recurent Implantation Failure;
BMI — body mass index;
PC — plasma cells;
hCG — human chorionic gonadotropin;
CE — chronic endometritis;
IVF — in vitro fertilization.
Примечание: таблица составлена авторами.
Сокращения:
CD — Cluster of Differentiation;
ERA — Endometrial Reciptivity Analysis;
ESPDI — индекс плотности стромальных плазмоцитов эндометрия
(endometrial stromal plasmacyte density index);
RIF — Reccurent Implantation Failure;
ИМТ — индекс массы тела;
ПК — плазматические клетки;
ХГЧ — хорионический гонадотропин человека;
ХЭ — хронический эндометрит;
ЭКО — экстракорпоральное оплодотворение.
Characteristics of studies included in the meta-analysis
The studies included a total of 4145 patients. All studies were observational: five prospective [9][18][43][44][46], five retrospective [6][12][14][17][45], and one crossover study [11].
Two studies compared non-CE patients with treated CE and recurrent CE patients [18][46]; one study compared women with treated and recurrent CE [6]; and two studies compared non-CE, treated CE, and infertile patients who were not tested for CE [9][17]. Three studies compared CE and non-CE patients [43][44][45], and one compared CE, non-CE, and treated CE patients [11]. Y. Li, S. Xu et al. (2021) divided patients into groups according to the number of plasma cells (0, 1, 3, 4, and ≥5 per HPF) and compared pregnancy outcomes in women with <5 and ≥5 CD138+ cells [12]. Also on the basis of PC count, Y. Xiong et al. (2021) compared pregnancy outcomes in patients with different CD138+ counts and recurrent CE following antibiotic therapy [14]. As the main criteria of CE recovery, T.M. Motovilova et al. chose sonographic markers—increase in endometrial thickness and blood flow change (resistance index, RI) in radial, spiral, and basal uterine arteries [43].
Four studies included patients with recurrent implantation failures (defined as failure of at least two or three previous IVF attempts (fresh or frozen/thawed embryos), including at least one high-quality cleavage-stage embryo or blastocyst transferred per cycle) [6][9][17][18][44]. One study included patients with only one previous embryo transfer failure [45], while four studies analyzed infertile patients with previous non-elective embryo transfers [11][12][14][44].
All of the women underwent IVF. Information on IVF protocols was missing in four studies [11][17][43][44], while the remaining seven provided adequate information on the protocols of assisted reproductive technologies (ART). Ovarian stimulation was conducted by administering recombinant FSH alone or in combination with human menopausal gonadotropin using GnRH. The administration of hCG was performed following visualization (transvaginal ultrasound) of at least two preovulatory (17 mm) follicles; oocyte retrieval was conducted 34–36 h following ovulation induction (transfer of no more than three embryos or two blastocysts): two studies transferred only cleavage-stage embryos (up to three) [6][46]; two studies transferred only blastocysts [11][44]; and two studies transferred both cleavage-stage embryos and blastocysts [14][17].
Two studies provided no data on the embryo stage [9][43]. Gestagen luteal phase support was carried out in all studies providing data on IVF protocols.
Risk of bias
All RCTs used randomization without allocation concealment—blinding of study participants and staff was not conducted due to patient specificity (infertility, in vitro fertilization protocol). Three studies had adequate sample representativeness [6][17][45], while the rest had a high risk of systematic error. Three studies reported an adequate (consistent) sampling strategy [11][17][18], whereas most studies provided no accurate data. In the diagnosis of chronic endometritis, all studies had a low risk of bias (≥3 points) (Table 2).
Two studies failed to clearly describe the study population or did not fully report descriptive statistics [18][46], while the others had a low risk of bias for this domain. Three studies provided incomplete data on outcomes [17][18][45]. According to the assigned points, all studies have a low risk of bias.
Table 2. Risk of bias assessment
Таблица 2. Оценка риска смещения
|
Authors and year |
Sample representativeness |
Sampling procedure |
Quality of patient descriptions |
Incomplete data on outcomes |
Total score |
Risk of bias |
|
Cicinelli et al. 2015 [6] |
* |
- |
* |
* |
**** |
low |
|
Demirdag et al. 2021 [17] |
* |
* |
* |
- |
**** |
low |
|
Fan et al. 2019 [45] |
* |
- |
* |
- |
*** |
low |
|
Hirata et al. 2021 [44] |
- |
- |
* |
* |
*** |
low |
|
John.-MacAnanny et al. 2010 [9] |
- |
- |
* |
* |
*** |
low |
|
Kitaya et al. 2017 [18] |
* |
* |
- |
- |
*** |
low |
|
Kuroda et al. 2020 [11] |
- |
* |
* |
* |
**** |
low |
|
Li et al. 2021 [12] |
* |
- |
* |
* |
**** |
low |
|
Xiong et al. 2021 [14] |
* |
- |
* |
* |
**** |
low |
|
Zhang et al. 2019 [46] |
* |
- |
- |
* |
*** |
low |
Note. The table was compiled by the authors.
Примечание: таблица составлена авторами.
Results of individual studies and their synthesis
Results of the meta-analysis of primary endpoints
Diagnosis of chronic endometritis
Plasma cell identification was achieved via hematoxylin and eosin (H&E) staining alone or in combination with immunohistochemical analysis of CD138+ cells, with the exception of the study that only provided an immunohistochemical analysis of PCs [43]. Six studies collected endometrial samples during the follicular phase in [6][14][18][44][45][46]; one study performed endometrial biopsies either in the follicular or mid-luteal phase (cycle days 21–23) [17]; two studies collected samples in the mid-luteal phase [11][12].
CE therapy
The first-line antibiotic therapy for CE was microbe-specific, with a microbiological culture assessment of the endometrium [6][11][46] or empiric (doxycycline [9][14][1][43] or ciprofloxacin and metronidazole for 14 days [18]).
Summary of the results
CE patients vs non-CE patients
Data from eight studies [9][11][12][14][17][18][44][46] showed significantly lower OPR/LBR (OR 1.97; 95% CI 1.11–3.48; I2=64%; p < 0.02) and CPR (OR 2.28; 95% CI 1.34–3.86; I2=70%; p < 0.002) in CE patients as compared to non-CE patients, with no difference in the MR (p < ns) (Tables 3–5; Figs. 2–4).
The sequential exclusion of each study from the meta-analysis provided no significant changes in the pooled results in terms of OPR/LBR, CPR, and MR.
Table 3. Ongoing pregnancy / live birth rate (CE patients vs. non-CE patients)
Таблица 3. Коэффициент частоты продолжающейся беременности/рождаемости
(хронический эндометрит и нехронический эндометрит)
|
|
non-CE |
CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Demirdag 2021 [17] |
31 |
103 |
36 |
129 |
20.2 |
1.11 (0.63, 1.97) |
|
J.-MacAnanny 2010 [9] |
12 |
23 |
1 |
10 |
5.2 |
9.82 (1.06, 9.59) |
|
Kitaya 2017 [18] |
50 |
226 |
0 |
4 |
3.3 |
2.58 (1.14, 8.63) |
|
Zhang 2019 [46] |
22 |
126 |
3 |
24 |
11.0 |
1.48 (0.41, 5.40) |
|
Hirata 2021 [44] |
14 |
27 |
6 |
26 |
12.1 |
3.59 (1.10, 11.73) |
|
Kuroda 2020 [11] |
18 |
27 |
3 |
18 |
9.4 |
10.00 (2.29, 4.73) |
|
Li 2021 [12] |
221 |
443 |
142 |
273 |
23.7 |
0.92 (0.68, 1.24) |
|
Xiong 2021 [14] |
42 |
83 |
8 |
26 |
15.1 |
2.05 (0.81, 5.22) |
|
Total (95% CI) |
1063 |
510 |
100.0 |
1.96 (1.11, 3.68) |
||
|
Total No. of cases |
410 |
199 |
||||
|
Heterogeneity: tau2=0.33; Chi2=15.43; df=7 (P < 0.007); I2=64% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Table 4. Clinical pregnancy rate (CE patients vs. non-CE patients)
Таблица 4. Коэффициент частоты клинической беременности
(хронический эндометрит и нехронический эндометрит)
|
|
non-CE |
CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Demirdag 2021 [17] |
39 |
103 |
47 |
129 |
16.4 |
1.06 (0.62, 1.82) |
|
Fan 2019 [45] |
78 |
97 |
23 |
44 |
13.8 |
3.75 (1.73, 8.14) |
|
J.-MacAnanny 2010 [9] |
12 |
23 |
2 |
10 |
6.2 |
4.36 (0.76, 25.17) |
|
Kitaya 2017 [18] |
61 |
226 |
0 |
4 |
2.8 |
3.34 (0.18, 63.03) |
|
Zhang 2019 [46] |
40 |
126 |
6 |
24 |
11.6 |
1.40 (0.51, 3.78) |
|
Hirata 2021 [44] |
17 |
27 |
8 |
26 |
10.2 |
3.83 (1.22, 11.98) |
|
Kuroda 2020 [11] |
21 |
27 |
4 |
18 |
8.0 |
12.25 (2.92, 51.42) |
|
Li 2021 [12] |
283 |
443 |
172 |
273 |
18.4 |
1.04 (0.76, 1.42) |
|
Xiong 2021 [14] |
58 |
88 |
11 |
26 |
12.6 |
2.64 (1.08, 6.45) |
|
Total (95% CI) |
1160 |
554 |
100.0 |
2.28 (1.34, 3.86) |
||
|
Total No. of cases |
609 |
273 |
||||
|
Heterogeneity: tau2=0.37; Chi2=26.24; df=8 (P < 0.0010); I2=70% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Table 5. Miscarriage rate (CE patients vs non-CE patients)
Таблица 5. Коэффициент частоты выкидышей
(хронический эндометрит и нехронический эндометрит)
|
|
non-CE |
CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Demirdag 2021 [17] |
8 |
39 |
11 |
47 |
18.9 |
0.84 (0.30, 2.36) |
|
J.-MacAnanny 2010 [9] |
0 |
12 |
1 |
2 |
1.5 |
0.04 (0.00, 1.50) |
|
Zhang 2019 [46] |
18 |
40 |
3 |
6 |
6.8 |
0.82 (0.15, 4.56) |
|
Hirata 2021 [44] |
3 |
17 |
2 |
8 |
4.9 |
0.64 (0.08, 4.89) |
|
Kuroda 2020 [11] |
3 |
21 |
1 |
4 |
3.0 |
0.50 (0.04, 6.55) |
|
Li 2021 [12] |
36 |
283 |
18 |
172 |
55.4 |
1.25 (0.68, 2.27) |
|
Xiong 2021 [14] |
16 |
58 |
3 |
11 |
9.6 |
1.02 (0.24, 4.32) |
|
Total (95% CI) |
470 |
250 |
100.0 |
0.99 (0.63, 1.54) |
||
|
Total No. of cases |
84 |
39 |
||||
|
Heterogeneity: tau2=0.00; Chi2=4.17; df=6 (P < 0.065); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
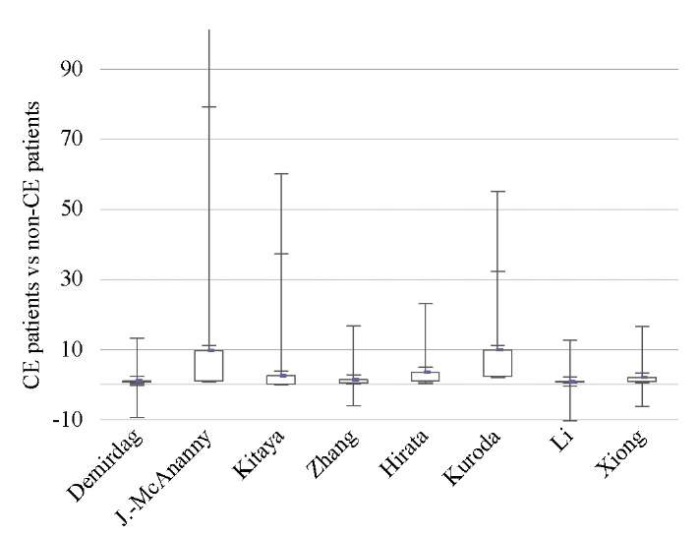
Fig. 2: Odds ratio (95% CI) (ongoing pregnancy / live birth rate)
Note: The figure was created by the authors.
Abbreviations:
ХЭ — chronic endometritis;
CI — confidence interval.
Рис. 2. Отношение шансов (95 % ДИ)
(частота продолжающейся беременности/рождаемости)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
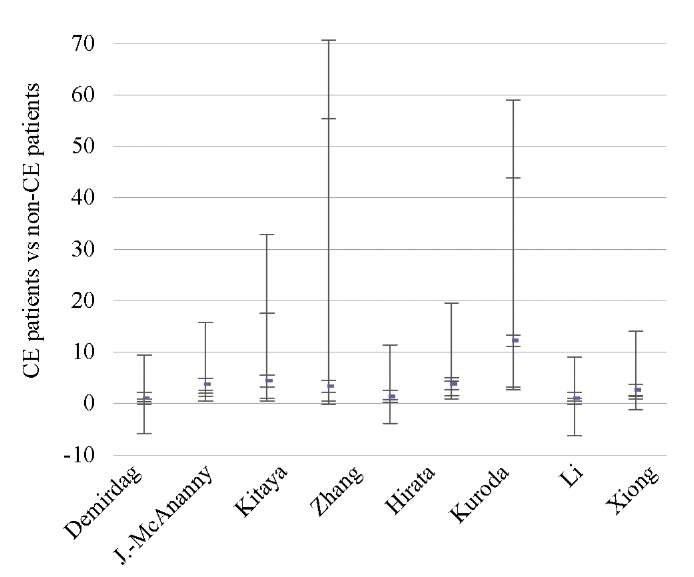
Fig. 3. Odds ratio (95% CI) (clinical pregnancy rate)
Note. The figure was created by the authors.
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Рис. 3. Отношение шансов (95 % ДИ)
(частота клинической беременности)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
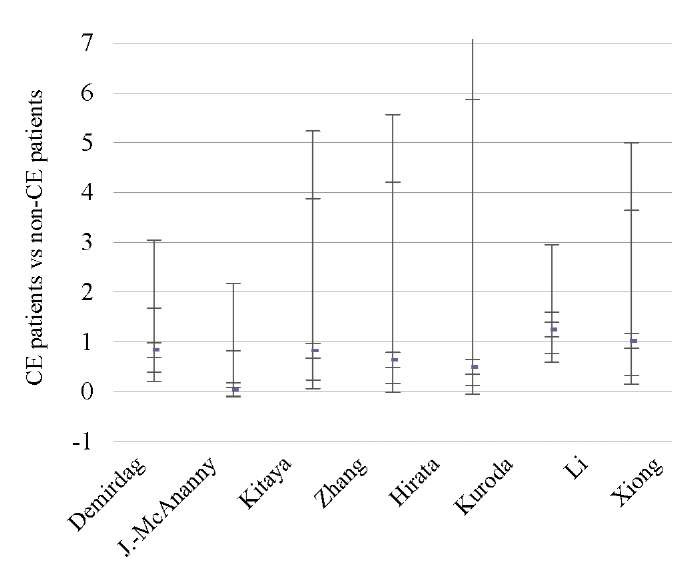
Fig. 4. Odds ratio (95% CI) (miscarriage rate)
Note. The figure was created by the authors.
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Рис. 4. Отношение шансов (95 % ДИ)
(коэффициент частоты выкидышей)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
CE patients vs treated CE patients
Four studies revealed higher OPR/LBR (OR 5.33; 95% CI 2.41–11.79; I2=0%; p < 0.0001) and CPR (OR 3.64; 95% CI 1.89–7.04; I2=0; p < 0.0001) in patients with treated CE as compared to patients with untreated/recurrent CE [6][11][18][46], with a borderline significance in terms of MR (p < 0.05) (Tables 6–8; Figs. 5–7). The sequential exclusion of individual studies from the meta-analysis provided no significant changes in the pooled results for OPR/LBR and CPR (sensitivity analysis was impossible for MR).
Table 6. Ongoing pregnancy / live birth rate (CE patients vs treated CE patients)
Таблица 6. Коэффициент частоты продолжающейся беременности/рождаемости
(хронический эндометрит и пролеченный хронический эндометрит)
|
|
Treated CE |
CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Cicinelli 2015 [6] |
28 |
46 |
2 |
15 |
24.5 |
10.11 (2.04, 50.19) |
|
Kitaya 2017 [18] |
25 |
116 |
0 |
4 |
7.3 |
5.73 (0.30, 98.91) |
|
Kuroda 2020 [11] |
11 |
29 |
3 |
18 |
30.0 |
3.06 (0.72, 13.01) |
|
Zhang 2019 [46] |
37 |
85 |
3 |
24 |
38.2 |
5.40 (1.50, 19.47) |
|
Total (95% CI) |
276 |
61 |
100.0 |
5.33 (2.41, 11.79) |
||
|
Total No. of cases |
121 |
8 |
||||
|
Heterogeneity: tau2=0.00; Chi2=1.18; df=3 (P < 0.76); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Table 7. Clinical pregnancy rate (CE patients vs treated CE patients)
Таблица 7. Коэффициент частоты клинической беременности
(хронический эндометрит и пролеченный хронический эндометрит)
|
|
Treated CE |
CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Cicinelli 2015 [6] |
30 |
46 |
5 |
15 |
28.5 |
3.75 (1.09, 12.87) |
|
Kitaya 2017 [18] |
53 |
116 |
0 |
4 |
5.0 |
7.58 (0.40, 94.05) |
|
Kuroda 2020 [11] |
15 |
29 |
4 |
18 |
24.6 |
3.75 (0.99, 14.16) |
|
Zhang 2019 [46] |
44 |
85 |
6 |
24 |
41.9 |
3.22 (1.16, 8.90) |
|
Total (95% CI) |
276 |
61 |
100.0 |
3.64 (1.89, 7.04) |
||
|
Total No. of cases |
142 |
15 |
||||
|
Heterogeneity: tau2=0.00; Chi2=0.30; df=3 (P < 0.96); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Table 8. Miscarriage rate (CE patients vs treated CE patients)
Таблица 8. Коэффициент частоты выкидышей
(хронический эндометрит и пролеченный хронический эндометрит)
|
|
Treated CE |
CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Cicinelli 2015 [6] |
2 |
30 |
3 |
5 |
31.2 |
0.05 (0.00, 0.47) |
|
Kuroda 2020 [11] |
4 |
15 |
1 |
4 |
27.3 |
1.09 (0.09, 13.78) |
|
Zhang 2019 [46] |
7 |
44 |
3 |
6 |
41.6 |
0.19 (0.03, 1.14) |
|
Total (95% CI) |
89 |
15 |
100.0 |
0.20 (0.04, 0.98) |
||
|
Total No. of cases |
13 |
7 |
||||
|
Heterogeneity: tau2=0.76; Chi2=3.22; df=2 (P < 0.20); I2=38% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
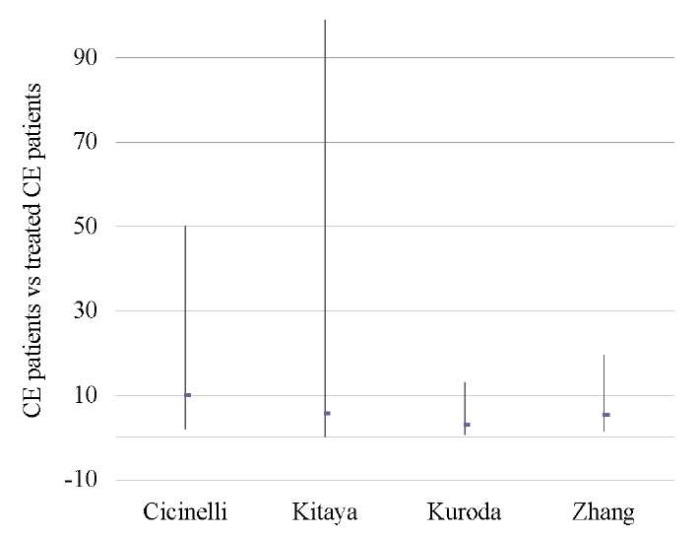
Fig. 5. Odds ratio (95% CI) (ongoing pregnancy / live birth rate)
Note. The figure was created by the authors.
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Рис. 5. Отношение шансов (95 % ДИ)
(Коэффициент частоты продолжающейся беременности/рождаемости)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
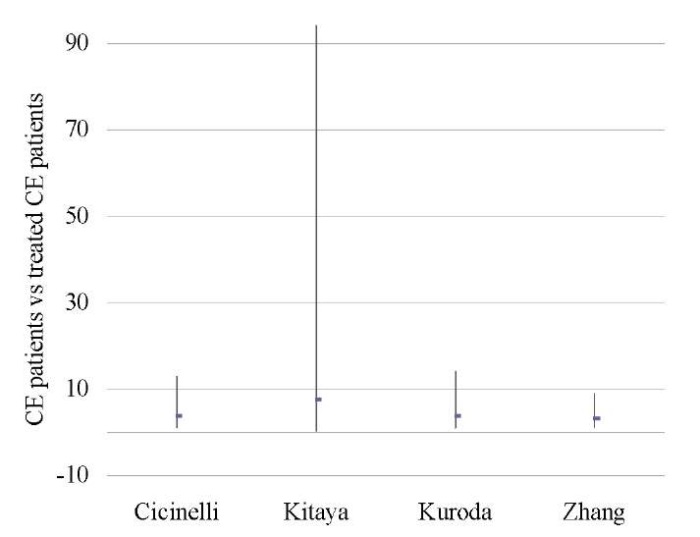
Fig. 6. Odds ratio (95% CI) (clinical pregnancy rate)
Note: The figure was created by the authors.
Abbreviations:
ХЭ — chronic endometritis;
CI — confidence interval.
Рис. 6. Отношение шансов (95 % ДИ)
(коэффициент частоты клинической беременности)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
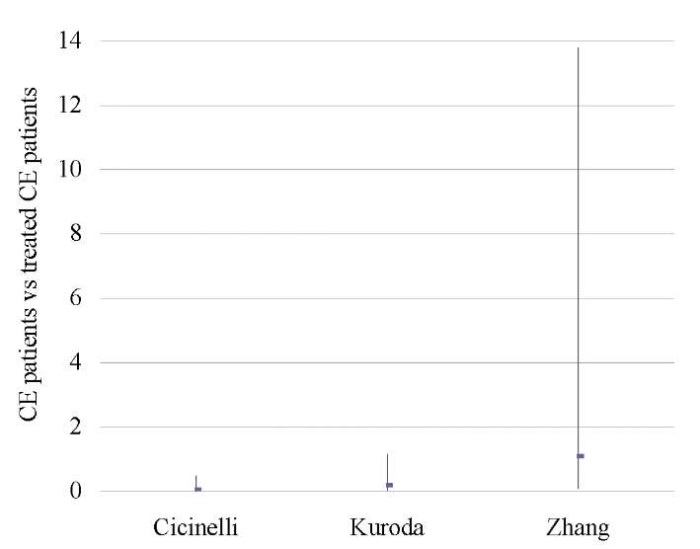
Fig. 7. Odds ratio (95% CI) (miscarriage rate)
Note. The figure was created by the authors.
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Рис. 7. Отношение шансов (95 % ДИ)
(коэффициент частоты выкидышей)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Treated CE patients vs non-CE patients
An analysis of 609 patients from three studies [11][18][46] showed no difference between the groups in terms of OPR/LBR, CPR, and MR (p < ns) (Tables 9–11; Figs. 8–10).
A sensitivity analysis was not possible due to the small number of studies included in the meta-analysis (n = 3).
Table 9. Ongoing pregnancy / live birth rate (non-CE patients vs treated CE patients)
Таблица 9. Коэффициент частоты продолжающейся беременности/рождаемости
(не-хронический эндометрит против пролеченного хронического эндометрита)
|
|
Treated CE |
non-CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Kitaya 2017 [18] |
38 |
116 |
50 |
226 |
36.6 |
1.71 (1.04, 2.82) |
|
Kuroda 2020 [11] |
11 |
29 |
18 |
27 |
28.4 |
0.31 (0.10, 0.91) |
|
Zhang 2019 [46] |
37 |
85 |
22 |
126 |
35.0 |
3.64 (1.94, 6.83) |
|
Total (95% CI) |
230 |
379 |
100.0 |
1.37 (0.46, 4.11) |
||
|
Total No. of cases |
86 |
90 |
||||
|
Heterogeneity: tau2=0.79; Chi2=14.91; df=2 (P < 0.0006); I2=87% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Table 10. Clinical pregnancy rate (non-CE patients vs treated CE patients)
Таблица 10. Коэффициент частоты клинической беременности
(не-хронический эндометрит против пролеченного хронического эндометрита)
|
|
Treated CE |
Non-CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Kitaya 2017 [18] |
43 |
116 |
61 |
226 |
39.0 |
1.59 (0.99, 2.57) |
|
Kuroda 2020 [11] |
15 |
29 |
21 |
27 |
24.0 |
0.31 (0.10, 0.98) |
|
Zhang 2019 [46] |
44 |
85 |
40 |
126 |
37.0 |
2.31 (1.31, 4.07) |
|
Total (95% CI) |
230 |
379 |
100.0 |
1.23 (0.53, 2.85) |
||
|
Total No. of cases |
102 |
122 |
||||
|
Heterogeneity: tau2=0.41; Chi2=9.36; df=2 (P < 0.009); I2=79% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Table 11. Miscarriage rate (non-CE patients vs treated CE patients)
Таблица 11. Коэффициент частоты выкидышей
(не-хронический эндометрит против пролеченного хронического эндометрита)
|
|
Treated CE |
Non-CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Kitaya 2017 [18] |
5 |
43 |
10 |
61 |
35.6 |
0.67 (0.21, 2.13) |
|
Kuroda 2020 [11] |
4 |
15 |
3 |
21 |
26.0 |
2.18 (0.41, 11.64) |
|
Zhang 2019 [46] |
7 |
44 |
18 |
40 |
38.4 |
0.23 (0.08, 0.64) |
|
Total (95% CI) |
102 |
122 |
100.0 |
0.61 (0.18, 1.99) |
||
|
Total No. of cases |
16 |
31 |
||||
|
Heterogeneity: tau2=0.69; Chi2=5.42; df=2 (P < 0.007); I2=63% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
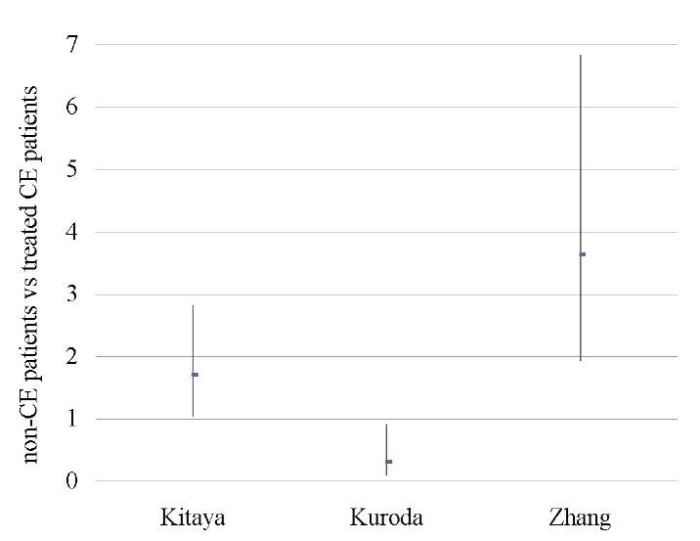
Fig. 8. Odds ratio (95% CI) (ongoing pregnancy / live birth rate)
Note: The figure was created by the authors.
Abbreviations:
ХЭ — chronic endometritis;
CI — confidence interval.
Рис. 8. Отношение шансов (95 % ДИ)
(коэффициент частоты продолжающейся беременности/рождаемости)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
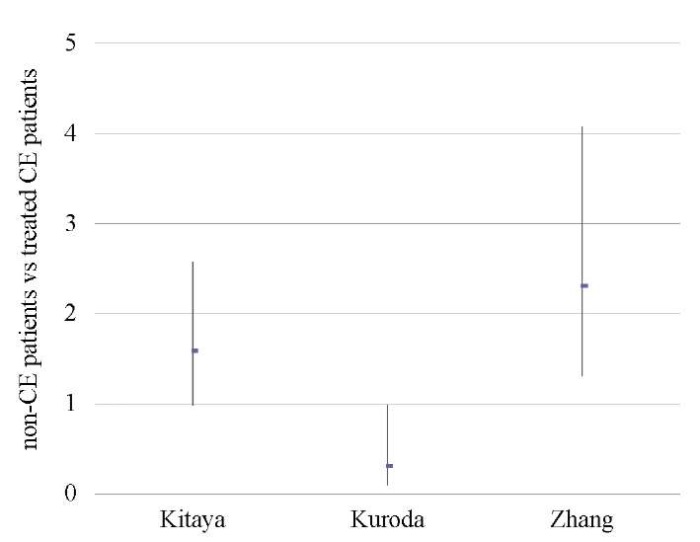
Fig. 9. Odds ratio (95% CI) (clinical pregnancy rate)
Note. The figure was created by the authors.
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Рис. 9. Отношение шансов (95 % ДИ)
(коэффициент частоты клинической беременности)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
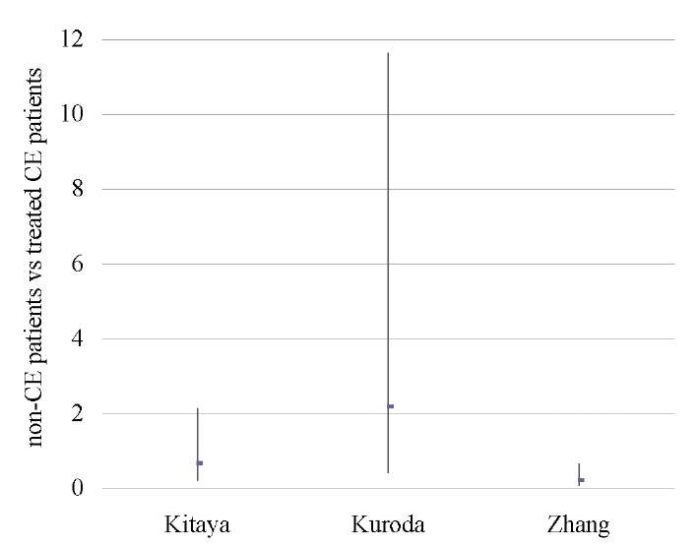
Fig. 10. Odds ratio (95% CI) (miscarriage rate)
Note. The figure was created by the authors.
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Рис. 10. Отношение шансов (95 % ДИ)
(коэффициент частоты выкидышей)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
CE patients vs patients who were not tested for this pathology
A pooled analysis of data on 1,556 patients from two studies [9][17] showed lower OPR/LBR (OR 0.55; 95% CI 0.37–0.82; I2=0%; p < 0.003) and CPR (OR 0.59; 95% CI 0.41–0.85; I2=0%; p < 0.005) in untreated CE patients as compared to those who was not tested for CE. No differences in miscarriage rates (p < ns) were found (Tables 12–14; Figs. 11–13).
A sensitivity analysis was not possible due to the small number of studies included in the meta-analysis (n = 2).
Table 12. Ongoing pregnancy / live birth rate (CE patients vs patients untested for CE)
Таблица 12. Коэффициент частоты продолжающейся беременности/рождаемости
(хронический эндометрит против нетестированного хронического эндометрита)
|
|
CE |
Untested for CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Demirdag 2021 [17] |
36 |
129 |
377 |
932 |
96.3 |
0.57 (0.38, 0.86) |
|
J.-MacAnanny 2010 [9] |
1 |
10 |
157 |
485 |
3.7 |
0.21 (0.03, 1.68) |
|
Total (95% CI) |
139 |
1417 |
100.0 |
0.55 (0.37, 0.82) |
||
|
Total No. of cases |
37 |
544 |
||||
|
Heterogeneity: tau2=0.00; Chi2=0.84; df=1 (P < 0.036); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Table 13. Clinical pregnancy rate (CE patients vs. patients untested for CE)
Таблица 13. Коэффициент частоты клинической беременности
(ХЭ против нетестированного ХЭ)
|
|
CE |
Untested for CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Demirdag 2021 [17] |
47 |
129 |
453 |
932 |
94.4 |
0.61 (0.38, 0.89) |
|
J.-MacAnanny 2010 [9] |
2 |
10 |
197 |
485 |
5.6 |
0.37 (0.08, 1.74) |
|
Total (95% CI) |
139 |
1417 |
100.0 |
0.59 (0.41, 0.85) |
||
|
Total No. of cases |
49 |
650 |
||||
|
Heterogeneity: tau2=0.00; Chi2=0.38; df=1 (P < 0.54); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Table 14. Miscarriage rate (CE patients vs patients untested for CE)
Таблица 14. Коэффициент частоты выкидышей
(хронический эндометрит против нетестированного хронического эндометрита)
|
|
CE |
Untested for CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Demirdag 2021 [17] |
11 |
47 |
76 |
453 |
93.8 |
1.52 (0.74, 3.11) |
|
J.-MacAnanny 2010 [9] |
1 |
2 |
20 |
197 |
6.2 |
5.57 (0.34, 91.44) |
|
Total (95% CI) |
49 |
650 |
100.0 |
1.64 (0.82, 3.30) |
||
|
Total No. of cases |
12 |
106 |
||||
|
Heterogeneity: tau2=0.00; Chi2=0.78; df=1 (P < 0.38); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
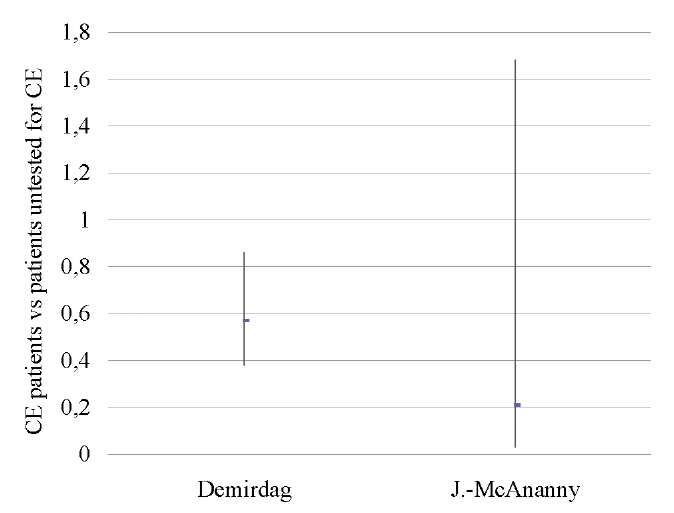
Fig. 11. Odds ratio (95% CI) (ongoing pregnancy / live birth rate)
Note: The figure was created by the authors.
Abbreviations:
ХЭ — chronic endometritis;
CI — confidence interval.
Рис. 11. Отношение шансов (95 % ДИ)
(коэффициент частоты продолжающейся беременности/рождаемости)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
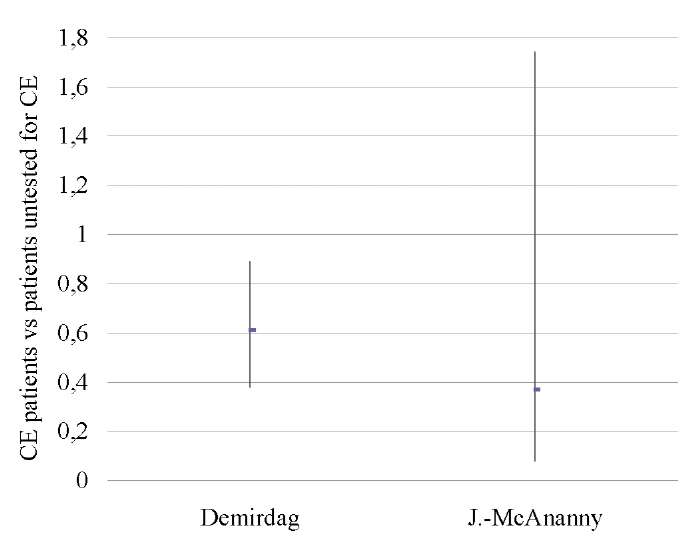
Fig. 12. Odds ratio (95% CI) (miscarriage rate)
Note. The figure was created by the authors.
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Рис. 12. Отношение шансов (95 % ДИ)
(коэффициент частоты выкидышей)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
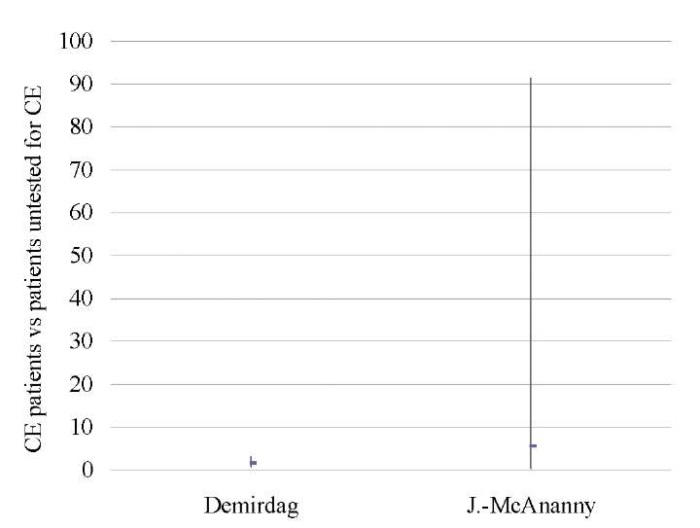
Fig. 13. Odds ratio (95% CI) (MR)
Note. The figure was created by the authors.
Abbreviations:
CE — chronic endometritis;
CI — confidence interval.
Рис. 13. Отношение шансов (95 % ДИ) (ЧВ)
Примечание: рисунок выполнен авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал.
Severe CE patients vs mild CE patients
Two studies [12][14] showed that severe CE (≥5 PCs per HPF) was associated with significantly lower OPR/LBR (OR 0.43; 95% CI 0.25–0.74; I2=0%; p < 0.003) and CPR (OR 0.40; 95% CI 0.24–0.68; I2=0%; p < 0.0007) as compared to mild CE (1–4 PCs per HPF), with no difference in the miscarriage rate (Table 15–17; Figs. 14–16).
A sensitivity analysis was not possible due to the small number of studies included in the meta-analysis (n = 2).
Table 15. Ongoing pregnancy/live birth rate (severe CE patients vs. mild CE patients)
Таблица 15. Коэффициент частоты продолжающейся беременности/рождаемости
(тяжелая степень хронического эндометрита
против легкой степени хронического эндометрита)
|
|
CE, ≥5 PCs |
CE, 1–4 PCs |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Li 2021 [12] |
15 |
36 |
127 |
206 |
58.6 |
0.44 (0.22, 0.91) |
|
Xiong 2021 [14] |
8 |
26 |
210 |
403 |
41.4 |
0.41 (0.17, 0.96) |
|
Total (95% CI) |
62 |
609 |
100.0 |
0.43 (0.25, 0.74) |
||
|
Total No. of cases |
23 |
377 |
||||
|
Heterogeneity: tau2=0.00; Chi2=0.02; df=1 (P < 0.88); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval;
PC — plasma cells.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал;
ПК — плазматические клетки.
Table 16. Clinical pregnancy rate (severe CE patients vs mild CE patients)
Таблица 16. Коэффициент частоты клинической беременности
(тяжелая степень хронического эндометрита
против легкой степени хронического эндометрита)
|
|
CE, ≥5 PCs |
CE, 1–4 PCs |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Li 2021 [12] |
17 |
38 |
155 |
235 |
57.3 |
0.42 (0.21, 0.84) |
|
Xiong 2021 [14] |
11 |
26 |
265 |
403 |
42.7 |
0.38 (0.17, 0.85) |
|
Total (95% CI) |
64 |
638 |
100.0 |
0.40 (0.24, 0.68) |
||
|
Total No. of cases |
28 |
420 |
||||
|
Heterogeneity: tau2=0.00; Chi2=0.03; df=1 (P < 0.87); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval;
PC — plasma cells.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал;
ПК — плазматические клетки.
Table 17. Miscarriage rate (severe CE patients vs. mild CE patients)
Таблица 17. Коэффициент частоты выкидышей
(тяжелая степень хронического эндометрита
против легкой степени хронического эндометрита)
|
|
CE, ≥5 PCs |
CE, 1–4 PCs |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Li 2021 [12] |
1 |
17 |
17 |
155 |
35.5 |
0.51 (0.06, 4.07) |
|
Xiong 2021 [14] |
3 |
11 |
38 |
265 |
64.5 |
2.24 (0.57, 8.82) |
|
Total (95% CI) |
28 |
420 |
100.0 |
1.32 (0.33, 5.32) |
||
|
Total No. of cases |
4 |
55 |
||||
|
Heterogeneity: tau2=0.29; Chi2=1.36; df=1 (P < 0.24); I2=27% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval;
PC — plasma cells.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал;
ПК — плазматические клетки.
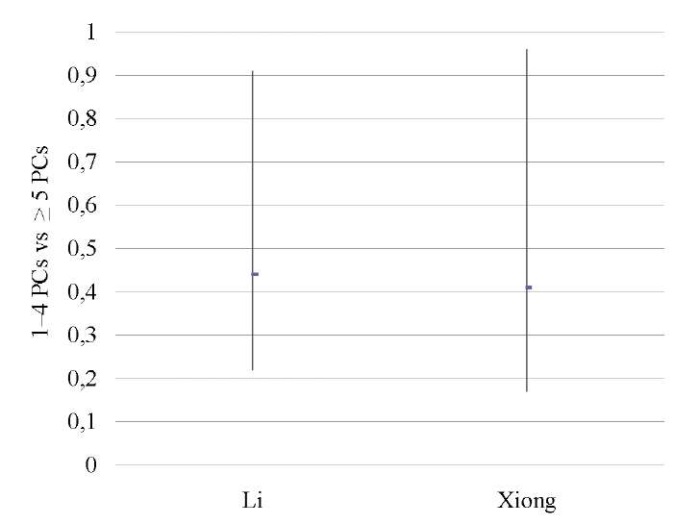
Fig. 14. Odds ratio (95% CI) (ongoing pregnancy/live birth rate)
Note: The figure was created by the authors.
Abbreviations:
ПК — plasma cells;
CI — confidence interval.
Рис. 14. Отношение шансов (95 % ДИ)
(коэффициент частоты продолжающейся беременности/рождаемости)
Примечание: рисунок выполнен авторами.
Сокращения:
ПК — плазматические клетки;
ДИ — доверительный интервал.
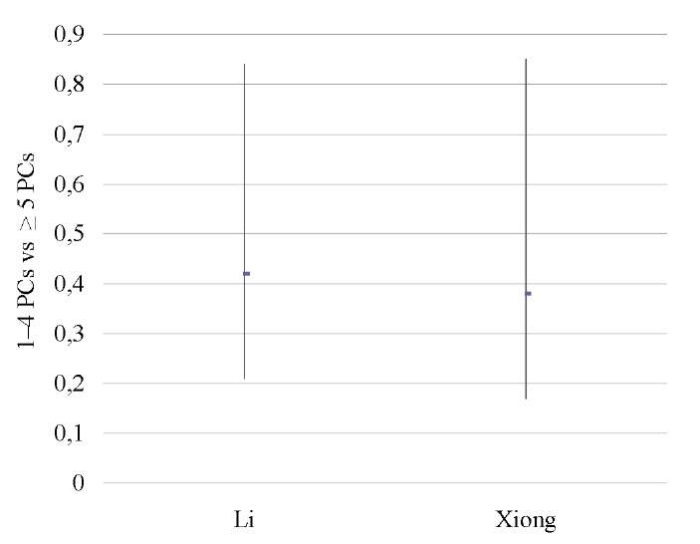
Fig. 15. Odds ratio (95% CI) (clinical pregnancy rate)
Note: The figure was created by the authors.
Abbreviations:
ПК — plasma cells;
CI — confidence interval.
Рис. 15. Отношение шансов (95 % ДИ)
(коэффициент частоты клинической беременности)
Примечание: рисунок выполнен авторами.
Сокращения:
ПК — плазматические клетки;
ДИ — доверительный интервал
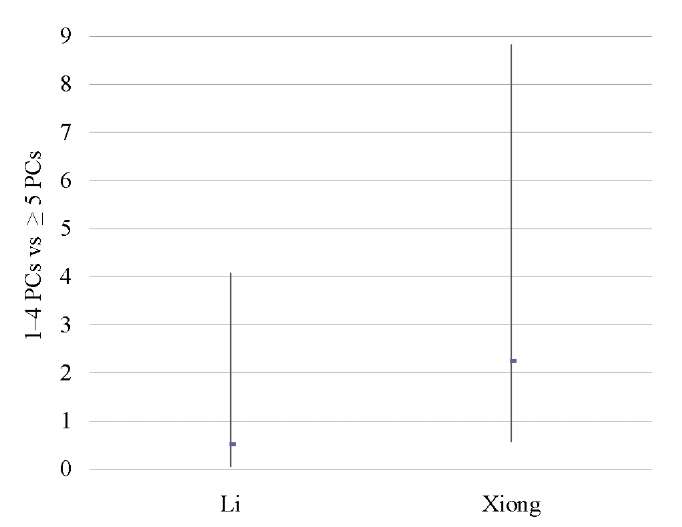
Fig. 16. Odds ratio (95% CI) (miscarriage rate)
Note: The figure was created by the authors.
Abbreviations:
ПК — plasma cells;
CI — confidence interval.
Рис. 16. Отношение шансов (95 % ДИ)
(коэффициент частоты выкидышей)
Примечание: рисунок выполнен авторами.
Сокращения:
ПК — плазматические клетки;
ДИ — доверительный интервал.
Mild CE patients vs patients untested for CE
No differences were found between the groups [12][14] in terms of OPR/LBR, CPR, and MR (p < ns) (Tables 18–20; Figs. 17–19).
A sensitivity analysis was not possible due to the small number of studies included in the meta-analysis (n = 2).
Table 18. Ongoing pregnancy / live birth rate
(mild CE patients vs patients untested for CE)
Таблица 18. Коэффициент частоты продолжающейся беременности/рождаемости
(легкая степень хронического эндометрита
против нетестированного хронического эндометрита)
|
|
CE, 1–4 PCs |
Non-CE
|
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Li 2021 [12] |
210 |
403 |
221 |
381 |
62.7 |
0.79 (0.59, 1.04) |
|
Xiong 2021 [14] |
104 |
199 |
42 |
88 |
37.3 |
1.20 (0.73, 1.98) |
|
Total (95% CI) |
602 |
469 |
100.0 |
0.92 (0.62, 1.37) |
||
|
Total No. of cases |
314 |
263 |
||||
|
Heterogeneity: tau2=0.05; Chi2=2.04; df=1 (P < 0.15); I2=51% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval;
PC — plasma cells.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал;
ПК — плазматические клетки.
Table 19. Clinical pregnancy rate (mild CE patients vs patients untested for CE)
Таблица 19. Коэффициент частоты клинической беременности
(легкая степень хронического эндометрита
против нетестированного хронического эндометрита)
|
|
CE, 1–4 PCs |
Non-CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Li 2021 [12] |
155 |
235 |
283 |
443 |
71.7 |
1.10 (0.79, 1.53) |
|
Xiong 2021 [14] |
131 |
199 |
58 |
88 |
28.3 |
1.00 (0.59, 1.69) |
|
Total (95% CI) |
434 |
531 |
100.0 |
0.40 (0.24, 0.68) |
||
|
Total No. of cases |
286 |
341 |
||||
|
Heterogeneity: tau2=0.00; Chi2=0.09; df=1 (P < 0.77); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval;
PC — plasma cells.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал;
ПК — плазматические клетки.
Table 20. Miscarriage rate (mild CE patients vs patients untested for CE)
Таблица 20. Коэффициент частоты выкидышей
(легкая степень хронического эндометрита
против нетестированного хронического эндометрита)
|
|
CE, 1–4 PCs |
Non-CE |
Study weight |
Odds ratio |
||
|
No. of cases |
Total No. of patients |
No. of cases |
Total No. of patients |
% |
95% CI |
|
|
Li 2021 [12] |
17 |
155 |
36 |
278 |
64.9 |
0.83 (0.45, 1.53) |
|
Xiong 2021 [14] |
15 |
131 |
12 |
58 |
35.2 |
0.50 (0.22, 1.14) |
|
Total (95% CI) |
286 |
336 |
100.0 |
0.69 (0.42, 1.13) |
||
|
Total No. of cases |
32 |
48 |
||||
|
Heterogeneity: tau2=0.00; Chi2=0.95; df=1 (P < 0.33); I2=0% |
||||||
Note. The table was compiled by the authors;
Abbreviations:
CE — chronic endometritis;
CI — confidence interval;
PC — plasma cells.
Примечание: таблица составлена авторами.
Сокращения:
ХЭ — хронический эндометрит;
ДИ — доверительный интервал;
ПК — плазматические клетки.
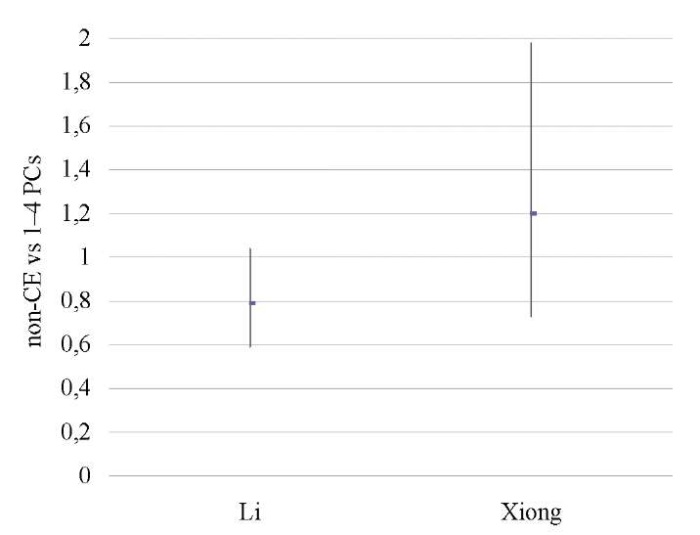
Fig. 17. Odds ratio (95% CI) (ongoing pregnancy / live birth rate)
Note. The figure was created by the authors.
Abbreviations:
PC — plasma cells;
CI — confidence interval.
Рис. 17. Отношение шансов (95 % ДИ)
(коэффициент частоты продолжающейся беременности/рождаемости)
Примечание: рисунок выполнен авторами.
Сокращения:
ПК — плазматические клетки;
ДИ — доверительный интервал.
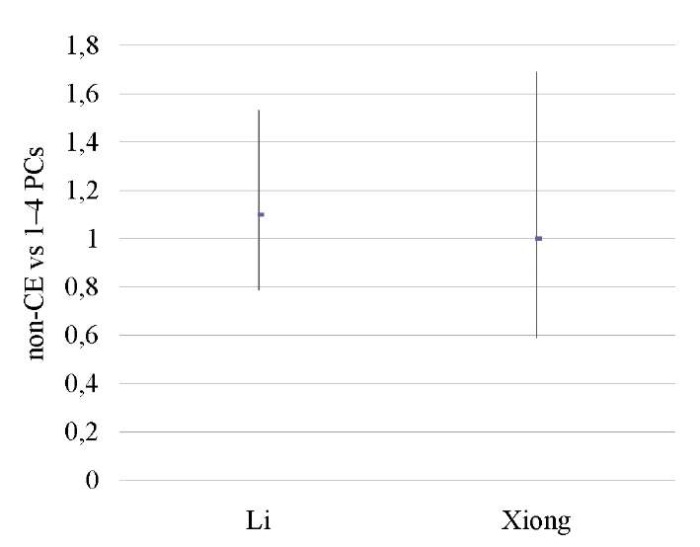
Fig. 18. Odds ratio (95% CI) (clinical pregnancy rate)
Note: The figure was created by the authors.
Abbreviations:
ПК — plasma cells;
CI — confidence interval.
Рис. 18. Отношение шансов (95 % ДИ)
(коэффициент частоты клинической беременности)
Примечание: рисунок выполнен авторами.
Сокращения:
ПК — плазматические клетки;
ДИ — доверительный интервал.
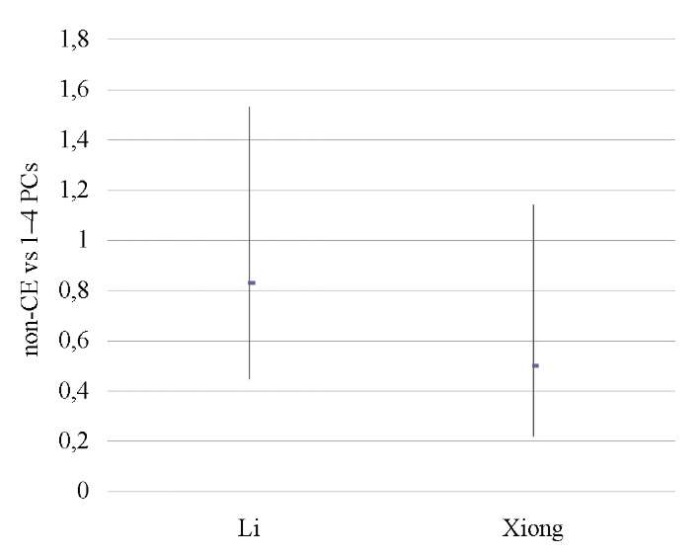
Fig. 19. Odds ratio (95% CI) (miscarriage rate)
Note: The figure was created by the authors.
Abbreviations:
ПК — plasma cells;
CI — confidence interval.
Рис. 19. Отношение шансов (95 % ДИ)
(коэффициент частоты выкидышей)
Примечание: рисунок выполнен авторами.
Сокращения:
ПК — плазматические клетки;
ДИ — доверительный интервал.
Risk of bias in all studies
Due to the limited number of included studies, publication bias could not be effectively represented using a funnel chart.
Additional analyses
No additional analyses were planned for this study.
DISCUSSION
Interpretation of results
The present systematic review summarizes the available data on the impact of chronic endometritis, its therapy, and its severity on the outcome of assisted reproductive technologies. The analysis included a total of 4,145 infertile patients from 11 studies [6][9][11][12][14][17][18][43][44][45][46]; of these, 1,716 were women with recurrent implantation failures. The overall quality of the included studies was considered satisfactory (no study had a high risk of bias).
Noteworthy is that non-CE women showed a significantly higher live birth rate (OPR/LBR) (OR 1.97; 95% CI 1.11–3.48) and clinical pregnancy rate (CPR) (OR 2.28; 95% CI 1.34–3.86) as compared to patients with diagnosed CE. This fact is consistent with the number of failed embryo transfers (p < 0.05) and supports the concept that CE has a negative effect on implantation [39], which is confirmed by a sufficiently large number of studies included in this meta-analysis, having equivalent approaches to CE diagnosis (i.e., CD138+ immunohistochemistry) [6][9][11][12][14][17][18][44][45][46].
Several major factors are currently considered responsible for reproductive dysfunction in CE [20]: this is primarily the abnormal uterine microbiome, as demonstrated by a sufficient number of microbiological studies [1][47–50], the disruption of cytobiochemical and immune processes in the endometrium [28][36][51–54], and also confirmed by the improved fertility following antibacterial therapy in CE patients [34][55][56]. The abnormal uterine microbiome leads to immune deviations primarily affecting the leukocyte pool of immunocompetent cells with a change in the ratio of pro- and anti-inflammatory cytokines, which results in the reduced implantation capacity of the endometrium and prevents embryo invasion [19][20][22][57]. In addition, prolonged activation of proliferation gene regulation with the downregulation of apoptotic genes (necessary for maintaining adequate endometrial responses) may contribute to the development of proliferative lesions such as polyps [25][34][55][58][61], whereas changes in the vascularization and decidualization of secretory endometrium in the presence of CE may additionally contribute to its receptivity impairment [9][59][60][62].
On the basis of data from two studies (which somewhat limits the value of their interpretation), patients with diagnosed CE were found to have worse IVF outcomes as compared to a group of infertile women who were not tested for this pathology (OPR/LBR: OR 0.55; 95% CI 0.37–0.82; CPR: OR 0.59; 95% CI 0.41–0.85; p < 0.05) [9][17]. If this result is confirmed by further studies, it could theoretically justify CE testing prior to IVF to identify (and treat) women with an expected poor reproductive prognosis. This is supported by the significant improvement in IVF outcomes following CE treatment that are presented in the review. It is true that the OPR/LBR and CPR were significantly higher following CE treatment as compared to those of patients with untreated or recurrent CE (OR 5.33; 95% CI 2.41–11.79; OR 3.64, 95% CI 1.89–7.04; p < 0.05). In addition, treated CE patients had similar IVF outcomes as women without this pathology (p>0.05), potentially indicating endometrial receptivity recovery after therapy.
Our conclusions for the MR seem to deviate from those for the other outcomes (OPR/LBR and CPR). The meta-analysis found neither a significant effect of CE on the miscarriage rate nor improvements in pregnancy outcomes in treated patients (p < 0.05). It can be assumed that spontaneous abortion involves too many different etiopathogenetic factors related to both the mother and the embryo which in this case reduces the role of endometrial inflammation [63–66]. In particular, aneuploidy is considered to be the main factor behind miscarriage, while maternal age (≥35 years) constitutes a major risk factor [61][67–70]. Most studies included in this meta-analysis had specific age limits: under 44 [12], 40 [44], 39 [6][14][17], and 38 years [45], while others (where age limits were not specified) reported mean patient ages close to [9] or above 35 years [11][18]. None of the studies conducted preimplantation genetic testing for aneuploidies. The only study conducted in women aged under 35 years showed a trend toward a higher miscarriage rate in CE patients as compared to healthy women or treated CE patients [46]. Also, we should note statistical problems associated with the sample size of patients taking into account the realization of their reproductive function and a high risk of error, as any comparative studies of miscarriage rate (MR) are inadequate as compared to the population-based estimation of CPR or OPR/LBR.
Unambiguous results were obtained when comparing the effect of severe (≥5 PCs per HPF) and mild (1–4 PCs per HPF) CE on IVF outcomes. Two studies showed that severe CE is associated with lower OPR/LBR (OR 0.43; 95% CI 0.25–0.74) and CPR (OR 0.40; 95% CI 0.24–0.68; p < 0.05) [12][14]. Of note is that mild CE patients exhibited similar results in terms of clinical pregnancy and live birth rates as women without this pathology (p>0.05). These data are consistent with the conclusions that a higher number of CD138+ cells determines a worse IVF outcome [12][45]. Although the possibility of categorizing CE by severity is attractive from a practical point of view, the available evidence is insufficient to consider “mild CE” as a condition that does not pose a significant threat to the implantation capacity of the endometrium [37]. CE classification that is based solely on plasma cell counts, although practical, is potentially prone to clinical errors due to the ambiguity of sampling methods—blind endometrial biopsy (pipelle biopsy or curettage), where the diagnosis may depend on the amount of captured endometrial tissue, especially if the distribution of PCs is heterogeneous. In addition, the severity of the process may be underestimated in the case of focal CE due to the random nature of morphological material collection from the uterine cavity. An equally important issue is that if PC counts are based solely on CD138+ staining, there is a possibility of overdiagnosis due to the background reaction of adjacent cells [34].
One of the most promising additional methods for diagnosing CE is hysteroscopy [6][16][32][34][65][71], especially in cases of diagnostic uncertainties [70][72][73]. Through visual evaluation of the entire endometrial surface, hysteroscopy can enable the identification of specific endometrial changes that are consistent with severe forms (e.g., micropolyps) [73][74]. In this connection, some studies indicate inconsistency between the CE diagnosis made on the basis of plasma cell counts and those made via hysteroscopy [6][22][32]. Therefore, we cannot exclude that the combination of the two methods may provide a higher diagnostic and prognostic value than immunohistochemistry alone. For example, in a study by Yang R. et al. (2014), patients whose control hysteroscopy showed no CE signs exhibited better IVF outcomes as compared to women whose recovery was assessed via immunohistochemistry (no PCs) [75]. Also, hysteroscopy is a technique that enables endometrial material sampling under visual control [76–78]. However, despite the fact that the use of hysteroscopy with biopsy is mandatory in focal endometrial lesions, its effectiveness is yet to be evaluated in CE diagnosis [15][79–83].
Study limitations
This meta-analysis has several limitations. The first is that the analysis is based on only ten RCTs and, therefore, a small number of women; thus, it is necessary to conduct more RCTs using a larger sample size. The second is the heterogeneity of patient characteristics (including IVF cycles and days of embryo transfer) included in the study—the variability of CE therapeutic regimens across studies and the inclusion of patients aged over 35 years without correction for the possibility of aneuploidy.
Meaning of the results
The evaluation of methods for diagnosing and treating chronic endometritis as one of the widespread factors in female infertility and, especially, implantation failures in assisted reproductive technologies, will help to promptly identify this pathology, conduct adequate therapy, and increase the effectiveness of in vitro fertilization programs.
CONCLUSION
Chronic endometritis can significantly reduce the effectiveness of in vitro fertilization in infertile women. Of note is that a rational antibacterial therapy of this pathology seems to improve the reproductive outcome, which becomes similar to that of women with no inflammatory changes in the endometrium.
The quality and number of studies on the limited negative impact of only severe CE (≥5 PCs per HPF) on IVF outcomes can currently be considered highly controversial, so further evidence is required.
Future RCTs are needed to check the effectiveness of the required CE tests in infertile patients prior to IVF in order to improve reproductive outcomes and increase live birth rates. In addition, further studies are recommended to evaluate the impact of chronic endometritis of varying severity on the IVF outcome and the feasibility of hysteroscopy in this pathology.
References
1. Moreno I, Cicinelli E, Garcia-Grau I, Gonzalez-Monfort M, Bau D, Vilella F, De Ziegler D, Resta L, Valbuena D, Simon C. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstet Gynecol. 2018;218(6):602.e1–602.e16. https://doi.org/10.1016/j.ajog.2018.02.012
2. Kitaya K, Takeuchi T, Mizuta S, Matsubayashi H, Ishikawa T. Endometritis: new time, new concepts. Fertil Steril. 2018;110(3):344–350. https://doi.org/10.1016/j.fertnstert.2018.04.012
3. Polovneva MI, Korneeva IE, Bourmenskaya OV. Modern methods of influence at endometrial receptivity in patients with recurrent implantation failure. Gynecology. 2018;20(3):66–70 (In Russ.) https://doi.org/10.26442/2079-5696_2018.3.66-70
4. Molina NM, Sola-Leyva A, Saez-Lara MJ, Plaza-Diaz J, Tubić-Pavlović A, Romero B, Clavero A, Mozas-Moreno J, Fontes J, Altmäe S. New Opportunities for Endometrial Health by Modifying Uterine Microbial Composition: Present or Future? Biomolecules. 2020;10(4):593. https://doi.org/10.3390/biom10040593
5. Remneva OV, Belnitskaya OA, Chernova AE, Yavorskaya SD. Chronic endometritis and infertility: correction by natural physical factors Altai. Mother and Child in Kuzbass. 2022;3(90):16–22 (In Russ.). https://doi.org/10.24412/2686-7338-2022-3-16-22
6. Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, Marrocchella S, Greco P, Resta L. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30(2):323–330. https://doi.org/10.1093/humrep/deu292
7. Kovalenko YaA, Chuprinenko LM. Structural and functional features of endometrium in patients undergoing in-vitro fertilization. Sechenov Medical Journal. 2019;10(1):29–34 (In Russ.). https://doi.org/10.26442/22187332.2019.1.29-34
8. Cicinelli E, Matteo M, Trojano G, Mitola PC, Tinelli R, Vitagliano A, Crupano FM, Lepera A, Miragliotta G, Resta L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am J Reprod Immunol. 2018;79(1). https://doi.org/10.1111/aji.12782
9. Chen P, Chen P, Guo Y, Fang C, Li T. Interaction Between Chronic Endometritis Caused Endometrial Microbiota Disorder and Endometrial Immune Environment Change in Recurrent Implantation Failure. Front Immunol. 2021;12:748447. https://doi.org/10.3389/fimmu.2021.748447.
10. Zargar M, Ghafourian M, Nikbakht R, Mir Hosseini V, Moradi Choghakabodi P. Evaluating Chronic Endometritis in Women with Recurrent Implantation Failure and Recurrent Pregnancy Loss by Hysteroscopy and Immunohistochemistry. J Minim Invasive Gynecol. 2020;27(1):116–121. https://doi.org/10.1016/j.jmig.2019.02.016
11. Zou Y, Li S, Ming L, Yang Y, Ye P, Zou J. The Correlation between Chronic Endometritis and Tubal-Factor Infertility. J Clin Med. 2022;12(1):285. https://doi.org/10.3390/jcm12010285.
12. Li Y, Xu S, Yu S, Huang C, Lin S, Chen W, Mo M, Lian R, Diao L, Ding L, Zeng Y. Diagnosis of chronic endometritis: How many CD138+ cells/HPF in endometrial stroma affect pregnancy outcome of infertile women? Am J Reprod Immunol. 2021;85(5):e13369. https://doi.org/10.1111/aji.13369
13. Orazov MR, Mikhaleva LM, Semenov PA. Chronic endometritis: pathogenesis, diagnosis, management and associated infertility. Clin. Exp. Morphology. 2020;9(2):16–25 (In Russ.). https://doi.org/10.31088/CEM2020.9.2.16-25
14. Xiong Y, Chen Q, Chen C, Tan J, Wang Z, Gu F, Xu Y. Impact of oral antibiotic treatment for chronic endometritis on pregnancy outcomes in the following frozen-thawed embryo transfer cycles of infertile women: a cohort study of 640 embryo transfer cycles. Fertil Steril. 2021;116(2):413–421. https://doi.org/10.1016/j.fertnstert.2021.03.036
15. Shamilova AM, Il’ina IYu, Borovkova EI, Dobrokhotova YuE. Chronic endometritis. Towards the improvement of diagnostic methods. Russian Journal of Woman and Child Health. 2021;4(3):243–249 (In Russ.). https://doi.org/10.32364/2618-8430-2021-4-3-243-249
16. Krasnopol’skaia KV, Nazarenko TA, Ershova IYu. Modern approaches to endometrial receptivity assessment (a review). Russian Journal of Human Reproduction. 2016;22(5):61–69 (In Russ.). https://doi.org/10.17116/repro201622561-69
17. Demirdag E, Guler I, Cevher Akdulum MF, Sahin E, Erdem O, Erdem A, Erdem M. Subsequent IVF outcomes following antibiotic therapy for chronic endometritis in patients with recurrent implantation failure. J Obstet Gynaecol Res. 2021;47(12):4350–4356. https://doi.org/10.1111/jog.15037
18. Kitaya K, Matsubayashi H, Takaya Y, Nishiyama R, Yamaguchi K, Takeuchi T, Ishikawa T. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol. 2017;78(5). https://doi.org/10.1111/aji.12719
19. Wang WJ, Zhang H, Chen ZQ, Zhang W, Liu XM, Fang JY, Liu FJ, Kwak-Kim J. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019;17(1):2. https://doi.org/10.1186/s12958-018-0444-9
20. Buzzaccarini G, Vitagliano A, Andrisani A, Santarsiero CM, Cicinelli R, Nardelli C, Ambrosini G, Cicinelli E. Chronic endometritis and altered embryo implantation: a unified pathophysiological theory from a literature systematic review. J Assist Reprod Genet. 2020;37(12):2897–2911. https://doi.org/10.1007/s10815-020-01955-8
21. Kabodmehri R, Etezadi A, Sharami SH, Ghanaei MM, Hosseinzadeh F, Heirati SFD, Pourhabibi Z. The association between chronic endometritis and uterine fibroids. J Family Med Prim Care. 2022;11(2):653–659. https://doi.org/10.4103/jfmpc.jfmpc_1470_21
22. Di Pietro C, Cicinelli E, Guglielmino MR, Ragusa M, Farina M, Palumbo MA, Cianci A. Altered transcriptional regulation of cytokines, growth factors, and apoptotic proteins in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2013;69(5):509–517. https://doi.org/10.1111/aji.12076
23. Wu D, Kimura F, Zheng L, Ishida M, Niwa Y, Hirata K, Takebayashi A, Takashima A, Takahashi K, Kushima R, Zhang G, Murakami T. Chronic endometritis modifies decidualization in human endometrial stromal cells. Reprod Biol Endocrinol. 2017;15(1):16. https://doi.org/10.1186/s12958-017-0233-x
24. Serebrennikova KG, Arutyunyan NA, Alekhin AI. Diagnosis and clinical criteria for chronic endometritis. Gynecology. 2018;20(6):53–59 (In Russ.). https://doi.org/10.26442/20795696.2018.6.180070
25. Cicinelli E, Vitagliano A, Loizzi V, De Ziegler D, Fanelli M, Bettocchi S, Nardelli C, Trojano G, Cicinelli R, Minervini CF, Leronni D, Viggiano L. Altered Gene Expression Encoding Cytochines, Grow Factors and Cell Cycle Regulators in the Endometrium of Women with Chronic Endometritis. Diagnostics (Basel). 2021;11(3):471. https://doi.org/10.3390/diagnostics11030471
26. Singh N, Sethi A. Endometritis — Diagnosis,Treatment and its impact on fertility — A Scoping Review. JBRA Assist Reprod. 2022;26(3):538–546. https://doi.org/10.5935/1518-0557.20220015
27. Kitaya K, Tada Y, Hayashi T, Taguchi S, Funabiki M, Nakamura Y. Comprehensive endometrial immunoglobulin subclass analysis in infertile women suffering from repeated implantation failure with or without chronic endometritis. Am J Reprod Immunol. 2014;72(4):386–391. https://doi.org/10.1111/aji.12277
28. Pinto V, Matteo M, Tinelli R, Mitola PC, De Ziegler D, Cicinelli E. Altered uterine contractility in women with chronic endometritis. Fertil Steril. 2015;103(4):1049–1052. https://doi.org/10.1016/j.fertnstert.2015.01.007
29. Yasuo T, Kitaya K. Challenges in Clinical Diagnosis and Management of Chronic Endometritis. Diagnostics (Basel). 2022;12(11):2711. https://doi.org/10.3390/diagnostics12112711
30. Farghali MM, Abdelazim IA, El-Ghazaly TE. Relation between chronic endometritis and recurrent miscarriage. Prz Menopauzalny. 2021;20(3):116–121. https://doi.org/10.1016/10.5114/pm.2021.109769
31. Khramtsova AYu, Bashmakova NV. Global view on the problem of «thin» endometrium: solutions to the problem in assisted reproductive technology (literature review). Russian Journal of Human Reproduction. 2019;25(4):69–76 (In Russ., In Engl.). https://doi.org/10.17116/repro20192504169
32. Cicinelli E, Vitagliano A, Kumar A, Lasmar RB, Bettocchi S, Haimovich S; International Working Group for Standardization of Chronic Endometritis Diagnosis. Unified diagnostic criteria for chronic endometritis at fluid hysteroscopy: proposal and reliability evaluation through an international randomized-controlled observer study. Fertil Steril. 2019;112(1):162–173.e2. https://doi.org/10.1016/j.fertnstert.2019.03.004
33. Mitter VR, Meier S, Rau TT, Gillon T, Mueller MD, Zwahlen M, von Wolff M, Kohl Schwartz AS. Treatment following hysteroscopy and endometrial diagnostic biopsy increases the chance for live birth in women with chronic endometritis. Am J Reprod Immunol. 2021;86(5):e13482. https://doi.org/10.1111/aji.13482
34. Cicinelli E, Haimovich S, De Ziegler D, Raz N, Ben-Tzur D, Andrisani A, Ambrosini G, Picardi N, Cataldo V, Balzani M, Cicinelli R, Noventa M, Marin L, Greco P, Resta L, Saccardi C, Buzzaccarini G, Vitagliano A; International Working Group for Standardization of Chronic Endometritis Diagnosis. MUM-1 immunohistochemistry has high accuracy and reliability in the diagnosis of chronic endometritis: a multi-centre comparative study with CD-138 immunostaining. J Assist Reprod Genet. 2022;39(1):219–226. https://doi.org/10.1007/s10815-02102356-1
35. Li Y, Yu S, Huang C, Lian R, Chen C, Liu S, Li L, Diao L, Markert UR, Zeng Y. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil Steril. 2020;113(1):187–196.e1. https://doi.org/10.1016/j.fertnstert.2019.09.001
36. Mihara M, Yasuo T, Kitaya K. Precision Medicine for Chronic Endometritis: Computer-Aided Diagnosis Using Deep Learning Model. Diagnostics (Basel). 2023;13(5):936. https://doi.org/10.3390/diagnostics13050936
37. Bouet PE, El Hachem H, Monceau E, Gariépy G, Kadoch IJ, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105(1):106–110. https://doi.org/10.1016/j.fertnstert.2015.09.025
38. Cicinelli E, Cicinelli R, Vitagliano A. Consistent evidence on the detrimental role of severe chronic endometritis on in vitro fertilization outcome and the reproductive improvement after antibiotic therapy: on the other hand, mild chronic endometritis appears a more intricate matter. Fertil Steril. 2021;116(2):345–346. https://doi.org/10.1016/j.fertnstert.2021.06.022
39. Vitagliano A, Saccardi C, Noventa M, Di Spiezio Sardo A, Saccone G, Cicinelli E, Pizzi S, Andrisani A, Litta PS. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and metaanalysis. Fertil Steril. 2018;110(1):103–112.e1. https://doi.org/10.1016/j. fertnstert.2018.03.017
40. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71
41. Vitagliano A, Laganà AS, De Ziegler D, Cicinelli R, Santarsiero CM, Buzzaccarini G, Chiantera V, Cicinelli E, Marinaccio M. Chronic Endometritis in Infertile Women: Impact of Untreated Disease, Plasma Cell Count and Antibiotic Therapy on IVF Outcome-A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2022;12(9):2250. https://doi.org/10.3390/diagnostics12092250
42. Cumpston MS, McKenzie JE, Welch VA, Brennan SE. Strengthening systematic reviews in public health: guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J Public Health (Oxf). 2022;44(4):e588–e592. https://doi.org/10.1093/pubmed/fdac036
43. Motovilova TM, Kachalina TS, Grechkanev GO, Gagaeva YuA. Clinical efficacy of bacteriophage and laser therapy in the treatment of chronic endometritis. Russian Journal of Human Reproduction. 2019;25(5):69–77 (In Russ.). https://doi.org/10.17116/repro20192505169
44. Hirata K, Kimura F, Nakamura A, Kitazawa J, Morimune A, Hanada T, Takebayashi A, Takashima A, Amano T, Tsuji S, Kaku S, Kushima R, Murakami T. Histological diagnostic criterion for chronic endometritis based on the clinical outcome. BMC Womens Health. 2021;21(1):94. https://doi.org/10.1186/s12905-021-01239-y. PMID: 33663485; PMCID: PMC7934457
45. Fan X, Li X, Li Y, Liao J, Chen H, Li Y, Lu GX, Lin G, Gong F. Endometrial CD138 count appears to be a negative prognostic indicator for patients who have experienced previous embryo transfer failure. Fertil Steril. 2019;112(6):1103–1111. https://doi.org/10.1016/j.fertnstert.2019.08.006
46. Zhang Y, Xu H, Liu Y, Zheng S, Zhao W, Wu D, Lei L, Chen G. Confirmation of chronic endometritis in repeated implantation failure and success outcome in IVF-ET after intrauterine delivery of the combined administration of antibiotic and dexamethasone. Am J Reprod Immunol. 2019;82(5):e13177. https://doi.org/10.1111/aji.13177
47. Moreno I, Simon C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod Med Biol. 2018;18(1):40–50. https://doi.org/10.1002/rmb2.12249
48. Lozano FM, Bernabeu A, Lledo B, Morales R, Diaz M, Aranda FI, Llacer J, Bernabeu R. Characterization of the vaginal and endometrial microbiome in patients with chronic endometritis. Eur J Obstet Gynecol Reprod Biol. 2021;263:25–32. https://doi.org/10.1016/j.ejogrb.2021.05.045
49. Franasiak JM. Chronic endometritis is associated with an altered microbiome, but what about treatment and clinical outcomes? Fertil Steril. 2019;112(4):649–650. https://doi.org/10.1016/j.fertnstert.2019.06.019
50. Mlodzik N, Lukaszuk K, Sieg W, Jakiel G, Smolarczyk R. Endometrial microbiota — do they mean more than we have expected? Ginekol Pol. 2020;91(1):45–48. https://doi.org/10.5603/GP.2020.0010
51. Baker JM, Chase DM, Herbst-Kralovetz MM. Uterine microbiota: residents, tourists, or invaders? Front Immunol. 2018;9:208. https://doi.org/10.3389/fimmu.2018.00208
52. Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Leber B, Horvath A, Pieber TR, Gorkiewicz G, Stadlbauer V, ObermayerPietsch B. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): A pilot study. PLoS One. 2017;12(1):e0168390. https://doi.org/10.1371/journal.pone.0168390
53. Cicinelli E, Cicinelli R, Vitagliano A. Antibiotic therapy for chronic endometritis and its reproductive implications: a step forward, with some uncertainties. Fertil Steril. 2021;115(6):14451446. https://doi.org/10.1016/j.fertnstert.2021.03.025
54. Song D, He Y, Wang Y, Liu Z, Xia E, Huang X, Xiao Y, Li TC. Impact of antibiotic therapy on the rate of negative test results for chronic endometritis: a prospective randomized control trial. Fertil Steril. 2021;115(6):1549–1556. https://doi.org/10.1016/j.fertnstert.2020.12.019
55. Cicinelli E, Bettocchi S, de Ziegler D, Loizzi V, Cormio G, Marinaccio M, Trojano G, Crupano FM, Francescato R, Vitagliano A, Resta L. Chronic endometritis, a common disease hidden behind endometrial polyps in premenopausal women: first evidence from a case-control study. J Minim Invasive Gynecol. 2019;26(7):1346–1350. https://doi.org/10.1016/j.jmig.2019.01.012
56. Kushnir VA, Solouki S, Sarig-Meth T, Vega MG, Albertini DF, Darmon SK, Deligdisch L, Barad DH, Gleicher N. Systemic inflammation and autoimmunity in women with chronic endometritis. Am J Reprod Immunol. 2016;75(6):672–677. https://doi.org/10.1111/aji.12508
57. Borovikov IO, Kravtsova EI, Bulgakova VP, Borovikova OI, Biryukova MI. Possibilities of correction of local immune status in patients with chronic endometritis. Medical Immunology (Russia). 2022. https://doi.org/10.15789/1563-0625-POC-2590
58. Vitagliano A, Cialdella M, Cicinelli R, Santarsiero CM, Greco P, Buzzaccarini G, Noventa M, Cicinelli E. Association between endometrial polyps and chronic endometritis: is it time for a paradigm shift in the pathophysiology of endometrial polyps in pre-menopausal women? Results of a systematic review and meta-analysis. Diagnostics (Basel). 2021;11(12):2182. https://doi.org/10.3390/diagnostics11122182
59. Ishida M, Takebayashi A, Kimura F, Nakamura A, Kitazawa J, Morimune A, Hanada T, Tsuta K, Murakami T. Induction of the epithelial-mesenchymal transition in the endometrium by chronic endometritis in infertile patients. PLoS One. 2021;16(4):e0249775. https://doi.org/10.1371/journal.pone.0249775
60. Gou J, Hu T, Li L, Xue L, Zhao X, Yi T, Li Z. Role of epithelialmesenchymal transition regulated by twist basic helix-loop-helix transcription factor 2 (Twist2) in embryo implantation in mice. Reprod Fertil Dev. 2019;31(5):932–940. https://doi.org/10.1071/RD18314
61. Ravel J, Moreno I, Simón C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am J Obstet Gynecol. 2021;224(3):251–257. https://doi.org/10.1016/j.ajog.2020.10.019
62. Maslova MA, Pavlovich SV, Smolnikova VYu. Current approaches to diagnosing and treating endometrial receptor apparatus disorders in patients with recurrent implantation failures. Akusherstvo i Ginekologiia. 2021;3:26–35 (In Russ,). http://dx.doi.org/10.18565/aig.2021.3.26-35
63. Vitagliano A, Noventa M, Gizzo S. Autoimmunity, systemic inflammation, and their correlation with repeated implantation failure and recurrent miscarriage: Is chronic endometritis the missing piece of the jigsaw? Am J Reprod Immunol. 2017;77(1). http://dx.doi.org/10.1111/aji.12597
64. Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11:154. http://dx.doi.org/10.1186/1741-7015-11-154
65. Krutova VA, Chulkova AM, Vanyan DL, Chuprinenko LM, Kasparyan RA, Kopytko EE, Dyakova TG. Neoadjuvant diagnosis and management of chronic endometritis. Kuban Scientific Medical Bulletin. 2018;25(1):23–29 (In Russ.). https://doi.org/10.25207/16086228-2018-25-1-23-29
66. Noventa M, Vitagliano A, Andrisani A, Blaganje M, Viganò P, Papaelo E, Scioscia M, Cavallin F, Ambrosini G, Cozzolino M. Testosterone therapy for women with poor ovarian response undergoing IVF: a meta-analysis of randomized controlled trials. J Assist Reprod Genet. 2019;36(4):673–683. https://doi.org/10.1007/s10815-018-1383-2
67. Tapilskaya NI, Tolibova GKh,Savicheva AM, Kopylova AA, Glushakov RI, Budilovskaya OV, Krysanova AA, Gorsky AG, Gzgzyan AM, Kogan IYu. The effectiveness of local cytokine therapy for chronic endometritis in patients with infertility. Akusherstvo i Ginekologiia. 2022;2:91–100 (In Russ,). http://dx.doi.org/10.18565/aig.2022.2.91-100
68. Padula F, Laganà AS, Vitale SG, D’Emidio L, Coco C, Giannarelli D, Cariola M, Favilli A, Giorlandino C. The introduction of the absolute risk for the detection of fetal aneuploidies in the first-trimester screening. J Matern Fetal Neonatal Med. 2017;30(10):1249–1253. http://dx.doi.org/10.1080/14767058.2016.1210123
69. Hirata K, Kimura F, Nakamura A, Kitazawa J, Morimune A, Hanada T, Takebayashi A, Takashima A, Amano T, Tsuji S, Kaku S, Kushima R, Murakami T. Histological diagnostic criterion for chronic endometritis based on the clinical outcome. BMC Womens Health. 2021;21(1):94. http://dx.doi.org/10.1186/s12905-021-01239-y
70. Drizi A, Djokovic D, Laganà AS, van Herendael B. Impaired inflammatory state of the endometrium: a multifaceted approach to endometrial inflammation. Current insights and future directions. Prz Menopauzalny. 2020;19(2):90–100. http://dx.doi.org/10.5114/pm.2020.97863
71. Borovikov IO, Kutsenko II, Bulgakova VP, Rubinina ER, Gorring HI, Voronov VA. Infertility against the background of chronic endometritis and vaginal dysbiosis: preimplantation preparation experience. Medical Council. 2020;3:115–121 (In Russ.). https://doi.org/10.21518/2079701X-2020-3-115-121
72. Puente E, Alonso L, Laganà AS, Ghezzi F, Casarin J, Carugno J. Chronic endometritis: old problem, novel insights and future challenges. Int J Fertil Steril. 2020;13(4):250-256. https://doi.org/10.22074/ijfs.2020.5779
73. Ellinidi VN, Feoktistov AA, Kazantsev VA, Obidniak DM. Prognostic value of two morphological forms of chronic endometritis: polypoid and follicular one. Russian Journal of Human Reproduction. 2020;26(1):55–58 (In Russ.). https://doi.org/10.17116/repro20202601155
74. Vitale SG, Haimovich S, Laganà AS, Alonso L, Di Spiezio Sardo A, Carugno J; From the Global Community of Hysteroscopy Guidelines Committee. Endometrial polyps. An evidence-based diagnosis and management guide. Eur J Obstet Gynecol Reprod Biol. 2021;260:70–77. https://doi.org/10.1016/j.ejogrb.2021.03.017
75. Yang R, Du X, Wang Y, Song X, Yang Y, Qiao J. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Arch Gynecol Obstet. 2014;289(6):1363–1369. https://doi.org/10.1007/s00404-013-3131-2
76. McQueen DB, Maniar KP, Hutchinson A, Confino R, Bernardi L, Pavone ME. Redefining chronic endometritis: the importance of endometrial stromal changes. Fertil Steril. 2021;116(3):855–861. https:// doi.org/10.1016/j.fertnstert.2021.04.036
77. Abdallah KS, Gadalla MA, Breijer M, Mol BWJ. Uterine distension media for outpatient hysteroscopy. Cochrane Database Syst Rev. 2021;11(11):CD006604. https://doi.org/10.1002/14651858.CD006604.pub2
78. Saccardi C, Vitagliano A, Marchetti M, Lo Turco A, Tosatto S, Palumbo M, De Lorenzo LS, Vitale SG, Scioscia M, Noventa M. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in post-menopausal women. Diagnostics (Basel). 2020;10(5):257. https://doi.org/10.3390/diagnostics10050257
79. Klimaszyk K, Svarre Nielsen H, Wender-Ozegowska E, Kedzia M. Chronic endometritis — is it time to clarify diagnostic criteria? Ginekol Pol. 2023;94(2):152–157. https://doi.org/10.5603/GP.a2022.0147.
80. Carugno J, Marbin SJ, LaganÀ AS, Vitale SG, Alonso L, DI Spiezio Sardo A, Haimovich S. New development on hysteroscopy for endometrial cancer diagnosis: state of the art. Minerva Med. 2021;112(1):12–19. https://doi.org/10.3390/10.23736/S0026-4806.20.07123-2
81. Luerti M, Vitagliano A, Di Spiezio Sardo A, Angioni S, Garuti G, De Angelis C; Italian School of Minimally Invasive Gynecological Surgery Hysteroscopists Group. Effectiveness of hysteroscopic techniques for endometrial polyp removal: the Italian multicenter trial. J Minim Invasive Gynecol. 2019;26(6):1169–1176. https://doi.org/10.3390/10.1016/j.jmig.2018.12.002
82. Murtinger M, Wirleitner B, Spitzer D, Bralo H, Miglar S, Schuff M. Diagnosing chronic endometritis: when simplification fails to clarify. Hum Reprod Open. 2022;2022(3):hoac023. https://doi.org/10.1093/hropen/hoac023
83. Kuroda K, Yamanaka A, Takamizawa S, Nakao K, Kuribayashi Y, Nakagawa K, Nojiri S, Nishi H, Sugiyama R. Prevalence of and risk factors for chronic endometritis in patients with intrauterine disorders after hysteroscopic surgery. Fertil Steril. 2022;118(3):568–575. https://doi.org/10.1016/j.fertnstert.2022.05.029
About the Authors
V. N. LokshinKazakhstan
Vyacheslav N. Lokshin — Dr. Sci. (Med.), Prof., academician, National Academy of Sciences, Republic of Kazakhstan; Director General, “Persona” International Clinical Center for Reproductive Medicine
Utepova str., 32a Almaty, 050000
I. I. Kutsenko
Russian Federation
Irina I. Kutsenko — Dr. Sci. (Med.), Prof., Head of the Obstetrics, Gynecology and Perinatology Department
Mitrofana Sedina str., 4, Krasnodar, 350063
I. O. Borovikov
Russian Federation
Igor O. Borovikov — Dr. Sci. (Med.), Assoc. Prof., Obstetrics, Gynecology and Perinatology Department
Mitrofana Sedina str., 4, Krasnodar, 350063
V. P. Bulgakova
Russian Federation
Vera P. Bulgakova — postgraduate student, Obstetrics, Gynecology and Perinatology Department
Mitrofana Sedina str., 4, Krasnodar, 350063
E. I. Kravtsova
Russian Federation
Elena I. Kravtsova — Cand. Sci. (Med.), Assoc. Prof., Obstetrics, Gynecology and Perinatology Department
Mitrofana Sedina str., 4, Krasnodar, 350063
M. I. Biryukova
Russian Federation
Maria I. Biryukova — postgraduate student, Obstetrics, Gynecology and Perinatology Department
Mitrofana Sedina str., 4, Krasnodar, 350063
O. I. Borovikova
Russian Federation
Olga I. Borovikova — postgraduate student, Obstetrics, Gynecology and Perinatology Department
Mitrofana Sedina str., 4, Krasnodar, 350063
J. V. Nikogda
Russian Federation
Julia V. Nikogda — Cand. Sci. (Med.), Teaching Assistant, Obstetrics, Gynecology and Perinatology Department
Mitrofana Sedina str., 4, Krasnodar, 350063
Review
For citations:
Lokshin V.N., Kutsenko I.I., Borovikov I.O., Bulgakova V.P., Kravtsova E.I., Biryukova M.I., Borovikova O.I., Nikogda J.V. Chronic endometritis and infertility — in vitro fertilization outcomes: systematic review and meta-analysis. Kuban Scientific Medical Bulletin. 2023;30(5):15-40. https://doi.org/10.25207/1608-6228-2023-30-5-15-40








































