Scroll to:
Morphological assessment of actin and desmin expression at different cold myocardial ischemia times: observational study
https://doi.org/10.25207/1608-6228-2024-31-1-15-26
Abstract
Background. Heart transplantation is currently the treatment of choice for patients in terminal stages of chronic heart failure. The critical shortage of donor organs and the growing need for heart transplantation necessitate the expansion of donor selection criteria, including the estimated ischemia time of the donor heart. Despite numerous studies, the issue remains regarding the safe cold ischemia time; no definite limit to the acceptable preservation time is known and no relevant pathomorphological data are available on the state of the donor heart myocardium at different time parameters. Objective. To comparatively assess the features of cardiomyocyte pathomorphology and expression of protein markers (actin and desmin) in the myocardium of a donor heart prior to the main stage of orthotopic heart transplantation. Methods. The work adopted the design of an observational clinical study, which was prospective in nature. The study used intraoperative myocardial biopsy specimens of the left atrial appendage from donors aged up to 60 years, following cold ischemia of the transplant in Bretschneider solution (Dr. Franz Köhler Chemie GmbH, Germany) lasting up to 240 minutes (Group 1, n = 10) and over 240 minutes (Group 2, n = 7). The nature of pathomorphological myocardial transformation in the left atrial appendage of the donor heart was determined at different cold ischemia times. Histological myocardial sections were stained with hematoxylin and eosin according to standard procedures. After that, they were further studied using light and polarization microscopy; the immunohistochemical method was used to analyze the expression of actin and desmin. Morphometry was performed using the ImageJ 1.48v software (USA). In the analysis of actin and desmin amount, the area of DAB(3,3′-diaminobenzidine)-positive products of the immunohistochemical reaction was estimated as a percentage of the image area. The volume density of immunohistochemically detectable actin and desmin was determined using 20 images at a magnification of 40×10. In order to study the intensity of the immune reaction, a semiquantitative method was used, which involved counting the number of cells in 25 randomly selected fields of view. The types of myocardial contracture damage were assessed via polarization microscopy. Results. Patients included in the first and second groups were comparable in terms of mean age and anthropometric indices. The mean age of patients amounted to 50 [44;59] years in Group 1 and 50 [49;50] years in Group 2, р = 0.193. The body mass index was 25 [22;27] in Group1 and 25 [21;31] in Group 2, р = 0.288. Both groups showed male predominance: 8 (80%) in Group 1 and 6 (85.7%) in Group 2, р = 0.256. The comprehensive morphological assessment of ischemic myocardial damage at different cold ischemia times revealed the uniformity and reversibility of changes in cellular structures (in both groups) that take the form of I–II class contractures, lysis changes in individual cardiomyocytes (only in Group 2), preserved immunohistochemical reactions to actin and desmin in both groups at their average intensity and the complete absence of areas showing no reaction to desmin, which gives an idea about the degree of preservation of their macromolecular structure. Conclusion. The obtained study results showed that due to having a balanced elemental composition that determines the metabolic protection of cells and their ionic balance, the Bretschneider solution effectively protects the donor heart during its transportation, with the myocardial cold ischemia lasting up to 240 min and more.
Keywords
For citations:
Kliver V.Е., Volkov A.M., Nadeev A.P., Fomichev A.V., Sirota D.A., Kliver E.Е., Zhulkov M.О., Pozdnyakova S.V. Morphological assessment of actin and desmin expression at different cold myocardial ischemia times: observational study. Kuban Scientific Medical Bulletin. 2024;31(1):15-26. https://doi.org/10.25207/1608-6228-2024-31-1-15-26
INTRODUCTION
One of the main problems faced by global health care today is terminal heart failure, for which heart transplantation remains the leading treatment [1]. The critical shortage of donor organs with the increasing need for heart transplantation indicates the inevitability of expanding donor selection criteria; among these, one of the most important criteria is the ischemia time of the donor heart. Increasing the ischemia time to over 240 minutes, according to some authors, raises the risk of developing transplant dysfunction and may lead to a lethal outcome [2][3].
Noteworthy is that normothermic ischemic myocardial damage is associated with the destructive effect of hypoxia on the structures of cellular proteins comprising the cellular framework of cardiomyocytes. Together with myosin, actin, which constitutes an integral part of microfilaments, creates the contractile elements of muscles, i.e., actomyosin complexes of sarcomeres. The reduction in sarcomeric actin intensity in the cytoplasm of muscle cells in the damaged areas indicates a decrease in the contractile function of cardiomyocytes1 [4]. Another representative of the main structural proteins of muscle cells is desmin [5][6], which binds cytoplasmic components creating a matrix near the sarcomere Z-disk and links it to the cytoplasmic zone of the cardiomyocyte membrane [7][8], thus linking both adjacent myofibrils and Z-disks [9][10]. In order to maintain longitudinal loading during contraction, desmin, through its own contact with the sarcomere binds the contractile part to the cell nucleus, organelles in the cell cytoplasm that provide cellular respiration, and the postsynaptic region [11][12]. This mechanism is most likely associated with the phosphorylation pathway disruption followed by the destruction of proteins in the cardiomyocyte cytoskeleton that are bound to them, as well as an increase in the number of calcium and hydrogen ions in the cells, which initiates calcium-dependent protease activity with the subsequent breakdown of actin and desmin.
Some studies report that prolonged cold ischemia of the transplant cannot be a factor in its dysfunction and has no effect on surgical outcomes [3][13]. The determination of myocardial damage using light optical microscopy is associated with certain difficulties if a short period of time has passed since its onset. In practice, it is reasonable to use an immunohistochemical method or polarized light microscopy in the diagnostics of myocardial damage [10][14–16], which provide a means to draw conclusions about the macromolecular structure status of cardiac muscle cells when studying areas of myocardial ischemia.
The article aims to comparatively assess the features of cardiomyocyte pathomorphology and expression of protein markers (actin and desmin) in the myocardium of donor hearts prior to the main stage of orthotopic heart transplantation.
METHODS
Study design
The work adopted the design of an observational clinical study, which was prospective in nature. Seventeen myocardial samples from the left atrial appendage (LAA) of donor hearts were included in the study.
Study conditions
The study was conducted at the Meshalkin National Medical Research Center, Ministry of Health of the Russian Federation. The heart transplantation procedure was carried out using a classical bicaval technique. Heart extraction was performed using the standard method, with preservation performed using a cold Bretschneider cardioplegic solution (Dr. Franz Köhler Chemie GmbH, Germany). In some cases, donor hearts from remote regions were used: Altai Krai and Kemerovo Oblast.
Eligibility criteria
Inclusion criteria
Hearts from donors aged up to 60 years. Absence of chest trauma, prolonged hypotension and hypoxemia; stable hemodynamics; mean arterial pressure (MAP) of >60 mmHg; central venous pressure (CVP) of 8–12 mmHg; inotropic support of below 10 mg/kg/min (dopamine); electrocardiography (ECG) and echocardiography (Echo-CG) revealing no pathological changes.
Exclusion criteria
Donors aged under 18. Complex cardiac rhythm disorders; need for excessive inotropic support (dopamine at a dose of 20 µg/kg/min or similar doses of other adrenergic preparations despite aggressive pre- and post-load optimization); contractile dysfunction according to echocardiography or left ventricular ejection fraction of < 40% despite the optimization of hemodynamics with inotropic drugs.
Removal criteria
Intravitam refusal of the patient to have internal organs removed.
Description of the eligibility criteria (diagnostic criteria)
The selection of donors for participation in the study involved establishing brain death on the basis of the clinical picture and results of laboratory and instrumental studies, which were conducted collegially in accordance with the national recommendations and accepted clinical protocols.2
Selection of group members
The participants were assigned to groups according to the time of preoperative cold ischemia of the transplant. The first group included ten cases with cold ischemia of the transplant lasting up to 240 minutes, while the second group consisted of seven cases with cold ischemia of the transplant lasting over 240 minutes.
Target parameters in the study
Main parameter in the study
The nature of pathomorphological myocardial rearrangements in the LAA of the donor heart was determined at different cold ischemia times.
Additional parameters in the study
Additional parameters are not provided in this study.
Methods for measuring the target parameters
In an operation theatre setting during the main stage of the operation, myocardial samples were collected from the LAA of the donor heart following cold ischemia with the use of Bretschneider solution (Dr. Franz Köhler Chemie GmbH, Germany). The fixation of myocardial slices, their histological processing, and subsequent hematoxylin-eosin staining were conducted according to available guidelines. The study of the obtained slide mounts was conducted by means of an Axio Scope.A1 universal microscope (Zeiss, Germany), equipped with an analyzer and polarizer, an AxioCam MRc5 camera (Zeiss, Germany), and ZEN blue software (Zeiss, Germany). The staining of myocardial slices for immunohistochemical analysis was performed according to the available recommendations of the antibody manufacturer and standards outlined in the guidelines for immunohistochemical studies [15]. The slices were dewaxed, and the tissue antigens were unmasked in a PT Link module (DAKO, Denmark) in citrate buffer (pH 6.0) at 95 °C for 60 minutes. Endogenous peroxidase was blocked with a 3% H2O2 solution, followed by protein blocking with a serum. After that, the obtained slices were incubated with antibodies to Actin, Muscle Specific Ab-4 (HHF35) and Desmin 43/GJA1 (clone D33, mouse monoclonal, DAKO, Denmark). Immunostaining was performed using a peroxidase-labeled polymer detection system (EnVision FLEX, DAKO, Denmark). At the final stage, cell nuclei were stained with hematoxylin.
Morphometry was performed using the ImageJ 1.48v software (USA). In the analysis of actin and desmin amount, the area of DAB-positive products of the immunohistochemical reaction was estimated as a percentage of the image area. The volume density (Vv,%) of immunohistochemically detectable actin and desmin was determined using 20 images at a magnification of 40×10. In order to study the intensity of the immune reaction, a semiquantitative method was used: (+/+++) counting the number of cells in 25 randomly selected fields of view; (-) no staining; (+) weak, only the cytoplasm of cells is stained; (++) moderate, with staining of cell cytoplasm and focal staining of extracellular components (<50%); (+++) marked expression, with staining of both cell cytoplasm and most of the extracellular components (>50%) [17]. The types of myocardial contracture damage were assessed via polarization microscopy [18].
Variables (predictors, confounders, and effect modifiers)
Previous congenital or acquired cardiac pathology could be a confounding factor independently affecting the study result. This factor was eliminated at the sampling stage by adding them to the exclusion criteria.
Statistical procedures
Principles behind sample size determination
The sample size was not determined in advance.
Statistical methods
A statistical analysis of the obtained results was performed using the STATISTICA 10.0 software (StatSoft, Inc., USA). Quantitative variables are presented as a median and interquartile range Me [Q1—Q3], while qualitative variables are given as the frequency of occurrence and/or percentage. An intergroup comparison of parameters was conducted using the Mann–Whitney test or Fisher’s exact test. The p-value of < 0.05 was considered statistically significant for all types of analysis.
RESULTS
Sampling
Two groups were formed for the study: Group 1 — ten cases with cold ischemia of the transplant lasting up to 240 minutes, i.e., 140 [ 140;150] minutes; Group 2 — seven cases with cold ischemia of the transplant lasting over 240 minutes, i.e., 375 [ 245;375] minutes (p ≤ 0.05) (Fig. 1).
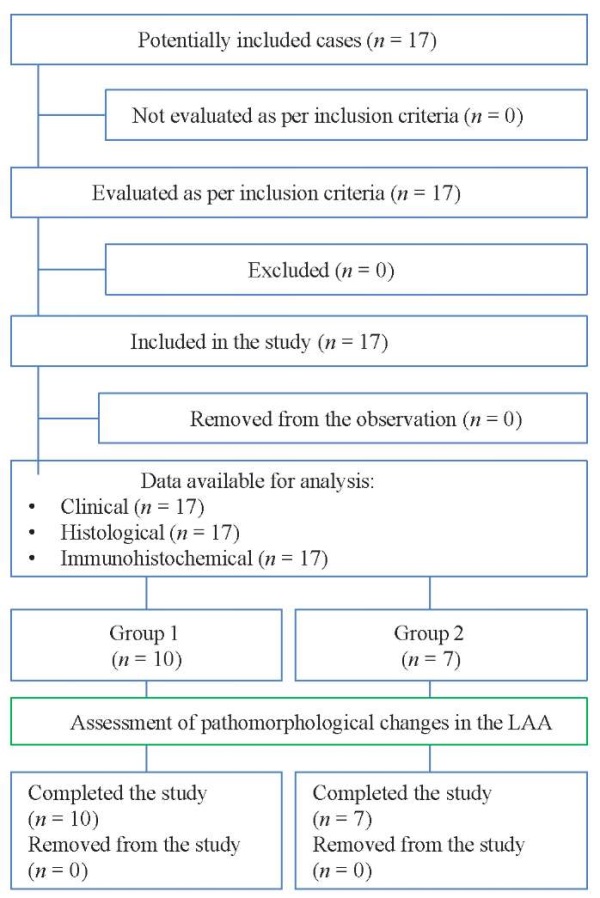
Fig. 1. Block diagram of the study design
Note: the block diagram was created by the authors (as per STROBE recommendations).
Abbreviation: LAA — left atrial appendage of the donor heart.
Рис. 1. Блок-схема дизайна проведенного исследования
Примечание: блок-схема выполнена авторами (согласно рекомендациям STROBE).
Сокращение: LAA — ушко левого предсердия донорского сердца.
Characteristics of the study sample (groups)
Patients included in the first and second groups were comparable in terms of mean age and anthropometric indices. The mean age of patients amounted to 50 [ 44;59] years in Group 1 and 50 [ 49;50] years in Group 2, р = 0.193. The body mass index (BMI) was 25 [ 22;27] in Group 1 and 25 [ 21;31] in Group 2, р = 0.288. Both groups showed male predominance: 8 (80%) in Group 1 and 6 (85.7%) in Group 2, р = 0.256.
Main study results
In the study of the material taken from the Group 1 patients, LAA myocardial preparations stained with hematoxylin and eosin revealed features characteristic of the fragmentation of individual muscle fibers in the stroma, as well as signs of non-uniform vascular plethora with moderate intermuscular edema and areas of erythrocytic sludges in microcirculatory vessels. A spasm of arterioles was noted, with veinous dilatation and plethora. Some vessels revealed a rod-shaped elongation of endotheliocytes. In general, most of the cardiomyocytes retained a regular structure with a slight sign of non-uniform oxyphilic staining characteristic of cells with myofibril contracture (Fig. 2).
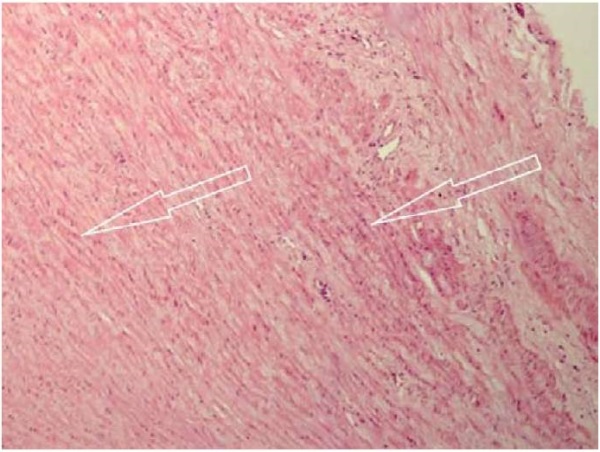
Fig. 2. Group 1 patients: cold ischemia of the transplant lasting up to 240 minutes.
Uneven eosinophilic staining of the left atrial myocardium (marked by arrows).
Hematoxylin and eosin staining, ×200
Note: the photograph was taken by the authors.
Рис. 2. Пациенты 1-й группы: холодовая ишемия трансплантата до 240 минут.
Неравномерность эозинофильной окраски миокарда левого предсердия
(отмечено стрелками).
Окраска гематоксилином и эозином. Увеличение ×200
Примечание: фотография выполнена авторами.
The use of polarization microscopy showed the presence of contractural changes in LAA muscle cells in both groups. Generally, such changes did not exceed subsegmental (contractions of individual groups of sarcomeres in the myofibrils of cardiomyocytes) and segmental Class I–II contractures (which involve the entire muscle cell) and were characterized by the increased anisotropy in the A-disk of sarcomeres with a varying degree of shortening of isotropic I-disks (Fig. 3). Cardiomyocytes having merged A-disks in the form of homogeneous anisotropy (Class III contracture) were observed sporadically in both groups.
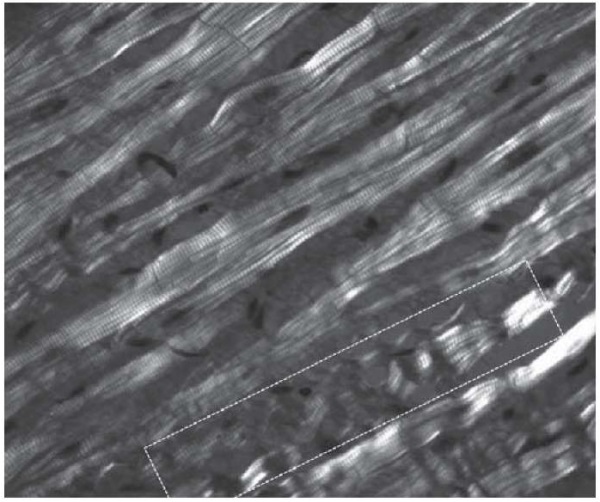
Fig. 3. Group 1 patients: cold ischemia of the transplant lasting up to 240 minutes.
Class I–II myofibril contractures.
Polarization microscopy (highlighted rectangle), ×630
Note: the photograph was taken by the authors.
Рис. 3. Пациенты 1-й группы: холодовая ишемия трансплантата до 240 минут.
Контрактуры миофибрилл I–II степени (выделено фигурой).
Поляризационная микроскопия. Увеличение ×630
Примечание: фотография выполнена авторами.
The study of actin and desmin expression revealed that most muscle cells were separated from each other by clearly outlined intercalated disks with preserved near-disk intensity in the form of dark-brown staining, which was revealed in the immunohistochemical reaction. In general, the cytoplasm had a uniform light brown color with increased reaction intensity in the Z-band region (Figs. 4 A, B). The preserved uniform staining of actin and desmin in cardiomyocytes both in the cytoplasm itself and in the region of intercalated disks, characterizing the sarcomeric cytoskeleton and the extra-sarcomeric cytoskeleton associated with the sarcolemma, indicates sufficient protection of the myocardium during prolonged ischemia. With unprotected myocardium, prolonged ischemia leads to the dissociation of the actin cytoskeleton from the cardiomyocyte membrane, followed by cell degeneration and apoptosis, which was not observed in the present study.
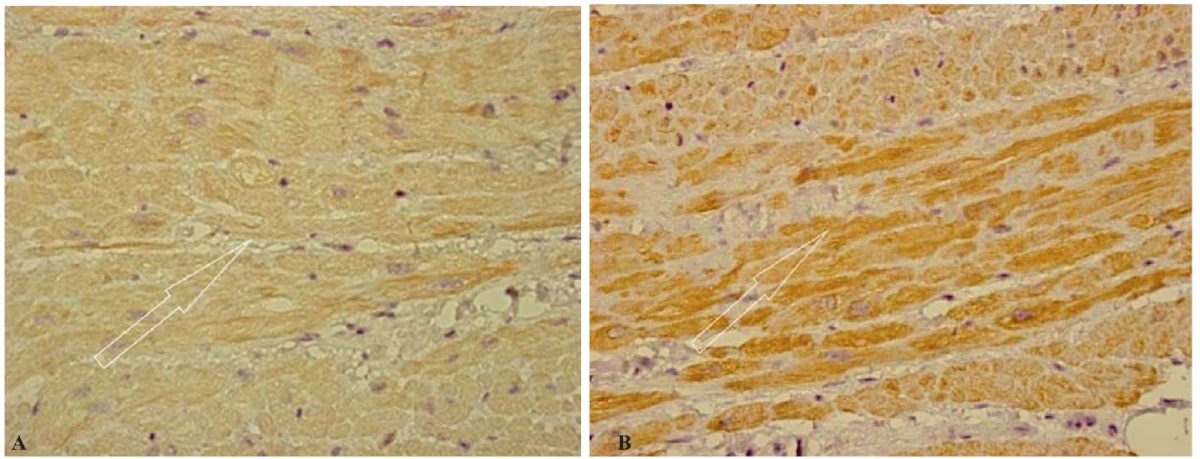
Fig. 4. Group 1 patients: cold ischemia of the transplant lasting up to 240 minutes.
Uniform immunohistochemical staining of actin (A)
and desmin (B) (marked by arrows) in cardiomyocytes.
Immunohistochemical study, ×400
Note: the photographs were taken by the authors.
Рис. 4. Пациенты 1-й группы: холодовая ишемия трансплантата до 240 минут.
Равномерное иммуногистохимическое окрашивание актина (А) (отмечено стрелкой)
и десмина (B) (отмечено стрелкой) в кардиомиоцитах.
Иммуногистохимическое исследование. Увеличение ×400
Примечание: фотографии выполнены авторами.
In the histological study of the material obtained from Group 2 patients with cold myocardial ischemia lasting over 240 minutes, dilation was observed in the perivascular spaces with wall edema of intramural arteries and preserved paretic changes in some of them, which involved venous plethora and dilation. Some arteries exhibited spastic changes with luminal narrowing; the endothelial lining retained its integrity. In microcirculatory vessels, erythrocytic sludges were observed in some places. Muscle fibers were mostly uniform in diameter with weak non-uniform oxyphilic staining of cardiomyocytes; only in some places, individual fibers were observed showing thinning and a wavy contour. Cytoplasmic edema was found in cardiomyocytes, which was accompanied by non-uniform staining. Along with the contractural changes of muscle cells, which did not exceed subsegmental and segmental Class I–II contractures, another type of change in cardiomyocytes—intracellular myocytolysis—was observed in polarized light. It was characterized by a decrease in the A-disk anisotropy or the absence of anisotropic structures within individual muscle cells due to the disappearance of one or another part of myofibrils. The preserved myofibrils were present as weakly anisotropic inclusions (Fig. 5).
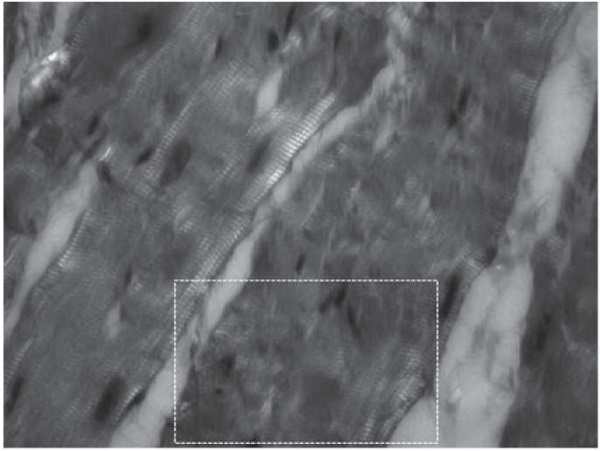
Fig. 5. Group 2 patients: cold ischemia of the transplant lasting over 240 minutes.
Foci of myocytolysis (highlighted rectangle).
Polarization microscopy, ×630
Note: the photograph was taken by the authors.
Рис. 5. Пациенты 2-й группы: холодовая ишемия трансплантата более 240 минут.
Очаги миоцитолизиса (обозначено фигурой).
Поляризационная микроскопия Увеличение ×630
Примечание: фотографии выполнены авторами.
In the immunohistochemical study, the expression of actin and desmin generally remained the same in the form of a light-brown staining reaction of cardiomyocyte cytoplasm while slightly increasing in some regions. In some fields of view, a decrease in intercalated disks was detected, with the emergence of deformations in some of them in the form of a “polyline band,” while a slight cross-striation blurring and moderate changes in the structure of the cytoplasm of muscle cells were observed in some places (Figs. 6 A, B).
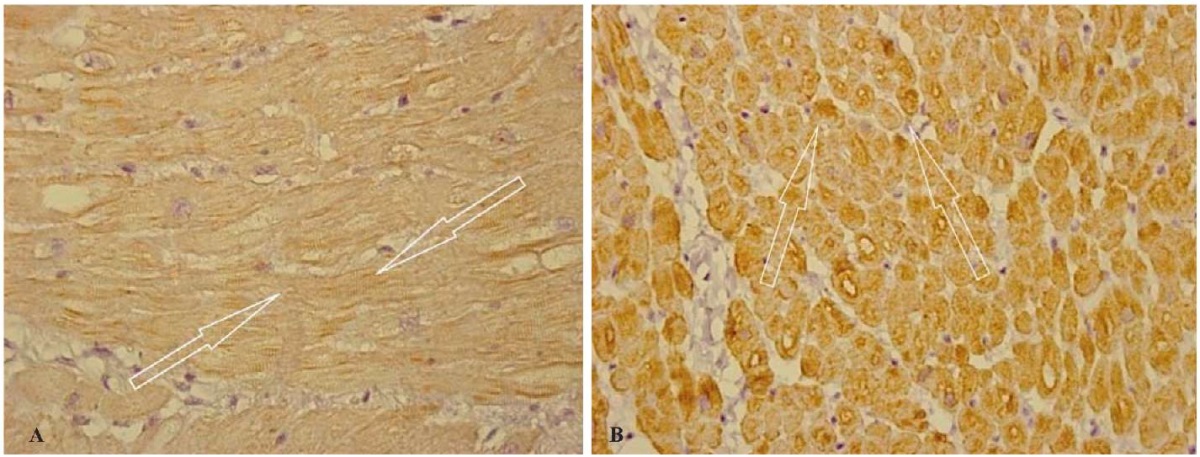
Fig. 6. Group 2 patients: cold ischemia of the transplant lasting over 240 minutes.
Uneven immunohistochemical staining of actin (A) (indicated by arrows)
and desmin (B) (indicated by arrows) in cardiomyocytes.
Immunohistochemical study, ×400
Note: the photographs were taken by the authors.
Рис. 6. Пациенты 2-й группы: холодовая ишемия трансплантата более 240 минут.
Неравномерное иммуногистохимическое окрашивание актина (А)
(обозначено стрелками)
и десмина (B) (обозначено стрелками) в кардиомиоцитах.
Иммуногистохимическое исследование. Увеличение ×400
Примечание: фотографии выполнены авторами.
Thus, the immunohistochemical analysis aimed at studying the expression of actin and desmin showed that in both studied groups, LAA cardiomyocytes exhibit a uniform light-brown staining reaction with its abrupt changes to dark-brown color in the region of the intercalated disks of muscle cells and throughout the muscle fiber in the region of Z-bands.
At different cold ischemia times (up to 240 minutes and over 240 minutes), fairly stable morphological dynamics of actin and desmin expression in the myocardium are observed according to the volume density parameter. The determination of this parameter in myocardial slices from the LAA in comparison of groups revealed its slight but statistically significant increase in Group 2: desmin, by 1.13 times; actin, by 1.07 times (Table), which may be associated with the continuing swelling of muscle cells due to the lysis changes in myofibrils, as well as with the absence of cellular-interstitial exchange during complete cold myocardial ischemia. In the comparison of two cold ischemia groups (up to 240 minutes and over 240 minutes) in terms of the intensity of actin and desmin expression, the morphological dynamics were reversed. With the parameter of immunohistochemically detectable actin and desmin increasing simultaneously, their expression intensities were reverse in nature. The intensity of desmin expression decreased by 1.6 times due to an increase in cardiomyocytes exhibiting a low level of intensity. Conversely, the intensity of actin expression increased by 1.5 times due to the increase in cardiomyocytes showing moderate and strong immunohistochemical reaction intensity (Table 1). For actin, this change in the expression intensity of the studied cellular proteins can be attributed to the contractile structure of the muscle cell—sarcomere, which is the main structural unit of the heart muscle and plays an important role in the effective regulation of cardiac rhythm and hemodynamic maintenance, while for desmin, to the involvement of compartments only beyond the sarcomeric cytoskeleton that provide physical support and structural integrity for heart cells.
Table. Volume density of actin and desmin and their expression intensity
in the cardiomyocytes of left atrial appendage
in patients with cold myocardial ischemia lasting up to 240 minutes (Group 1) and over 240 minutes (Group 2)
Таблица. Объемная плотность, интенсивность экспрессии кардиомиоцитами
ушка левого предсердия актина и десмина
у пациентов с холодовой ишемией миокарда до 240 минут (1-я группа)
и более 240 минут (2-я группа)
|
Parameter |
Group 1 (n = 10) |
Group 2 (n = 7) |
|
Immunohistochemical parameters of the LAA myocardium |
||
|
Desmin, Vv, % |
73 [ 69;76] |
83 * [ 79;86] |
|
Desmin, expression intensity (%) |
+/++/+++ 1/68/31 |
+/++/+++ 39/58/3 |
|
Actin, Vv, % |
78 [ 74;81] |
84 * [ 69;76] |
|
Actin, expression intensity (%) |
+/++/+++ 35/52/13 |
+/++/+++ 4/78/18 |
Notes: the table was compiled by the authors;
* — differences in the corresponding values between the groups (р < 0,05);
+/++/+++ –weak/moderate/strong expression intensity;
Vv — volume density.
Примечания: таблица составлена авторами;
* — отличия от соответствующих значений между группами (р < 0,05);
+/++/+++ — слабая/умеренная/выраженная интенсивность экспрессии;
Vv — объемная плотность структур.
Additional study results
No additional results were obtained during the study.
DISCUSSION
Main findings of the study
The comprehensive morphological state assessment of LAA myocardium showed stabile actin and desmin expression in patients with cold ischemia lasting up to 240 minutes and more. Structural changes developing in the form of contractures did not exceed Class I–II. Lysis changes were noted in the individual cardiomyocytes of Group 2 patients.
Research limitations
The results of the present study were obtained on small samples of patients. In order to further develop the completed work, a study using a larger sample size is required.
Interpretation of the study results
Several works [19–22] examine the nature of the cellular response in case of damage to the molecular structures of the cell that realize the interaction of its membrane with intracellular elements comprising the cytoskeleton structure. The structure of cardiomyocytes, including the arrangement of cellular proteins in it, is strictly determined. Cellular proteins are polyfunctional, performing a variety of functions and interacting with different structures in the extracellular and intracellular space. This allows them to ensure that the cell remains preserved and stable during cardiac cycles. By interacting with the extracellular matrix components, cellular proteins provide mechanical strength and support to cells and help in the formation and stabilization of tissue structures. Within the cell, proteins interact with various organelles, thus regulating cellular metabolism, cellular energy supply, protein transport and sorting, as well as signaling pathways within the cell [21–26]. In literary sources, heart diseases involving damage to the structure of muscle cells are mostly cardiomyopathies [23]. The use of the immunohistochemical method in diagnosing damage to the molecular structures of the cell enables assessment of both the dynamics of changes in their number and the degree of cardiomyocyte integrity.
The analysis of actin and desmin expression in the study of myocardium during cold ischemia showed that they are reversed in the groups. The intensity of desmin expression decreased with increasing ischemia time (on the point scale, the group with one point characteristic significantly increased) (Table 1). A significant decrease in desmin expression in various forms of ischemic myocardial damage was reported in a large number of studies. Ischemic myocardial damage leads to impaired blood supply to the heart and subsequent cardiomyocyte death. This can cause changes in the expression of different proteins, including desmin. Studies using immunohistochemistry, immunoblotting, and other methods also confirm that ischemic myocardial damage results in decreased desmin expression, which is associated with the activation of pathological signaling pathways, altered gene expression, and other mechanisms. Decreased desmin expression in ischemic myocardial damage has important implications for myocardial structure and function. Under these conditions, a decrease in desmin level leads to impaired cell integrity, as well as the disorganization of sarcomeres and other structural components. However, of note is that the nature and extent of changes in desmin expression may differ at different stages and in different forms of ischemic myocardial damage. For example, I.V. Zadnipryanyi et al. (2017) report a significant decrease in desmin expression in various forms of ischemic myocardial damage while noting the its heterogeneous presence in different regions—from no response of cardiomyocytes to desmin to its strengthening in the region of Z-bands and intercalated disks [27].
With prolonged complete ischemia of the LAA myocardium, the present study also showed different expression intensities during cold ischemia in both groups; however, no regions exhibiting no reaction to desmin were observed. The expression intensity of actin, unlike that of desmin, increased in Group 2 patients (Table 1) and was non-uniform. Taking into account that during complete cold myocardial ischemia, the repair of damaged myofibrils via activation of synthetic processes is impossible, another mechanism behind enhancing actin expression should be assumed, which is associated with the increased availability of reactive groups in actin filaments under conditions of protein denaturation, with the development of mixed damages in the form of contractures and myocytolysis [19]. This may also be attributed to the inclusion of additional receptors during the fragmentation of contractures, spirally twisted actin filaments in foci of myofibril overcontraction and overstretching.
CONCLUSION
The study revealed that the use of Bretschneider solution during cold myocardial ischemia effectively protects the donor heart during its transportation (with the myocardial cold ischemia lasting up to 240 min and more) due to its balanced elemental composition that determines the metabolic protection of cells and their ionic balance. The comprehensive morphological state assessment of the LAA myocardium in patients with cold ischemia lasting up to 240 minutes and more, which involved the use of light and polarization microscopy and immunohistochemical studies, showed stable actin and desmin expression in the myocardium, which indicates the reversibility of structural changes in the form of contractures not exceeding Class I–II. Lysis changes in individual cardiomyocytes (only in Group 2), as well as preserved expression of actin and desmin in both groups at their average intensity, indicate a sufficiently high degree of preservation of their macromolecular structure in both groups to restore adequate cardiac function following heart transplantation.
1. Zbrueva YuV, Kul’bitskii BN, Kabakova SS, Dzhuvalyakov PG, Bogomolov DV, Fedulova MV. Immunohistochemical study of heart contusion. Sudebno-Meditsinskaya Ekspertisa. 2013;56(1):54–55.
2. Ministry of Health of the Russian Federation, Order No. 908n of December 25, 2014 “On the Procedure for Diagnosing Human Brain Death”.
References
1. Gautier SV. Innovations in transplantology: heart transplantation program development in Russian Federation. Circulation Pathology and Cardiac Surgery. 2017;21(3S):61-68 (In Russ.). https://doi.org/10.21688/1681-3472-2017-3S-61-68
2. Poptsov VN, Zakharevich VM, Spirina EA, Koloskova NN, Pchelnikov VV, Khatutskii VM, Skokova AI, Fomichev AV, Aliev EZ, Bo-ronova VA, Bereznyak AV, Solodovnikova AK. Perioperative period in heart transplantation with extremely prolonged ischemic times (>6 hours). Russian Journal of Transplantology and Artifi cial Organs. 2022;24(3):64-73 (In Russ.). https://doi.org/10.15825/1995-1191-2022-3-64-73
3. Tenchurina EA, Minina MG. Mo dern ideas in heart donor selection criteria. Russian Journal of Transplantology and Artifi cial Organs. 2020;22(3):174-181 (In Russ.). https://doi.org/10.15825/1995-1191-2020-3-174-181
4. Kupryte M, Lesauskaite V, Keturakis V, Buneviciene V, Utkiene L, Jusiene L, Pangonyte D. Remodeling of Cardiomyocytes: Study of Morphological Cellular Changes Preceding Symptomatic Ischemic Heart Failure. Int J Mol Sci. 2023;24(19):14557. https://doi.org/10.3390/ijms241914557
5. Singh SR, Kadioglu H, Patel K, Carrier L, Agnetti G. Is Desmin Propensity to Aggregate Part of its Protective Function? Cells. 2020;9(2):491. https://doi.org/10.3390/cells9020491
6. Charrier EE, Montel L, Asnacios A, Delort F, Vicart P, Gallet F, Ba-tonnet-Pichon S, Henon S. The desmin network is a determinant of the cytoplasmic stiffness of myoblasts. Biol Cell. 2018;110(4):77-90. https://doi.org/10.1111/boc.201700040
7. Even C, Abramovici G, Delort F, Rigato AF, Bailleux V, de Sousa Moreira A, Vicart P, Rico F, Batonnet-Pichon S, Briki F. Mutation in the Core Structure of Desmin Intermediate Filaments Affects Myoblast Elasticity. Biophys J. 2017;113(3):627-636. https://doi.org/10.1016/j.bpj.2017.06.020
8. Agnetti G, Herrmann H, Cohen S. New roles for desmin in the maintenance of muscle homeostasis. FEBS J. 2022;289(10):2755-2770. https://doi.org/10.1111/febs.15864
9. Hnia K, Ramspacher C, Vermot J, Laporte J. Desmin in muscle and associated diseases: beyond the structural function. Cell Tissue Res. 2015;360(3):591-608. https://doi.org/10.1007/s00441-014-2016-4
10. Sokolova OV. Forensic medical characteristics of metabolic myocardial lesions affecting cardiac contractile ability in cases of death from alcoholic cardiomyopathy. Sudebno-Meditsinskaya Ekspertisa. 2021;64(5):27-31 (In Russ.). https://doi.org/10.17116/sudmed20216405127
11. Kiss B, Rohlich P, Kellermayer MS. Structure and elasticity of desmin protofibrils explored with scanning force microscopy. J Mol Recognit. 2011;24(6):1095-1104. https://doi.org/10.1002/jmr.1158
12. Kreplak L, Herrmann H, Aebi U. Tensile properties of single desmin intermediate filaments. Biophys J. 2008;94(7):2790-2799. https://doi.org/10.1529/biophysj.107.119826
13. Fomichev AV, Poptsov VN, Sirota DA, Zhulkov MO, Edemskiy AG, Protopopov AV, Kliver VY, Skokova AI, Chernyavskiy AM, Khvan DS, Agayeva KA. Mid-term and long-term outcomes following heart transplantation with prolonged cold ischemia. Russian Journal of Transplantology and Artifi cial Organs. 2023;25(1):99-105. https://doi.org/10.15825/1995-1191-2023-1-99-105
14. Sokolova OV, Yagmurov OD, Nasyrov RA. Forensic medical assessment of morphological changes in the myocardium, affecting its contractile capacity in cases of death from alcoholic cardiomyopathy. Pe-diatr. 2018;9(1):23-28 (In Russ). https://doi.org/10.17816/PED9123-28
15. Savchenko SV, Novoselov VP, Morozova AS, Skrebov RV, Grizinger VA, Ageeva TA, Aidagulova SV, Erschov K.I., Voronina E.I. Assessment of the severity of connexin 43 expression in the myocardium in acute ischemia in experiment. Circulation Pathology and Cardiac Surgery. 2017;21(1):81-90 (In Russ.). https://doi.org/10.21688/1681-3472-2017-1-81-90
16. Chu P, Weiss L. Modern Immunohistochemistry. P. Chu, L. Weiss, editors. 2016. https://doi.org/10.1017/cbo9781316167366
17. Liskova YV. Pathogenetic and prognostic significance of molecular markers of myocardial remodeling in patients suffering from chronic heart failure. Bulletin of the Russian Military Medical Academy. 2018;20(1):19-23. https://doi.org/10.17816/brmma12195
18. Nepomnyashchikh LM, Zimmermann VG. Prenecrotic contracture damage in cardiomyocytes: photochemical fluorochrome staining and fluorescent microscopy of the myocardium. Bull Exp Biol Med. 2001;132(3):907-913. https://doi.org/10.1023/a:1013147524226
19. Kolyubaeva SN, Kachnov VA, Tyrenko VV, Chirsky VS, Protasov OV, Onishchenko LS, Buntovskaya AS, Myakoshina LA, Ermilova IV, Eliseeva MI. A study of the morphological features of the heart muscle in sudden cardiac death. Vestnik Natsional'nogo mediko-khirurgichesko-go Tsentra im. N.I. Pirogova. 2020;15(3):31-35 (In Russ.). https://doi.org/10.25881/BPNMSC.2020.40.75.006
20. Deev RV, Bilyalov AI, Zhampeisov TM. Modern ideas about cell death. Geny i Kletki. 2018;13(1):8-19 (In Russ.). https://doi.org/10.23868/201805001
21. Savchenko SV, Novoselov VP, Skrebov RV, Grebenshchikova AS, Gritsinger VA, Ageeva TA, Voronina EI, Kazanskaya GM, Ovsyanko EV. Evaluation of protein changes of the myocardium in acute ischemia according to the immunohistochemical study. Journal of Siberian Medical Sciences. 2019;3:95-104 (In Russ.). https://doi.org/10.31549/2542-1174-2019-3-95-104
22. Ammendolia DA, Bement WM, Brumell JH. Plasma membrane integrity: implications for health and disease. BMC Biol. 2021;19(1):71. https://doi.org/10.1186/s12915-021-00972-y
23. Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodou-lou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lak-dawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Se-idman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366(7):619-628. https://doi.org/10.1056/NEJMoa1110186
24. Bar H, Strelkov SV, Sjoberg G, Aebi U, Herrmann H. The biology of desmin filaments: how do mutations affect their structure, assembly, and organisation? J Struct Biol. 2004;148(2):137-152. https://doi.org/10.1016/j.jsb.2004.04.003
25. Shi SR, Shi Y, Taylor CR, Gu J. New Dimensions of Antigen Retrieval Technique: 28 Years of Development, Practice, and Expansion. Appl Immunohistochem Mol Morphol. 2019;27(10):715-721. https://doi.org/10.1097/PAI.0000000000000778
26. Pantin-Jackwood MJ. Immunohistochemical Staining of Influenza Virus in Tissues. Methods Mol Biol. 2020;2123:29-36. https://doi.org/10.1007/978-1-0716-0346-8_3
27. Zadnipr yanyi IV, Sataeva TP, Tretyakova OS. Morphological markers for nitrite-induced myocardial damage in an experiment. Russian Journal of Operative Surgery and Clinical Anatomy. 2017;1(2):7-12 (In Russ.). https://doi.org/10.17116/operhirurg2017127-12
About the Authors
V. Е. KliverRussian Federation
Vladislav Е. Kliver - Research Assistant, Research Department of Surgery on Aorta, Coronary and Peripheral Arteries, Institute of Circulatory Pathology, Meshalkin National Medical Research Center, Ministry of Health of the Russian Federation; postgraduate student, Anatomic Pathology Department, Novosibirsk State Medical University, Ministry of Health of the Russian Federation.
Rechkunovskaya str., 15, Novosibirsk 600055; Krasny Prospect, 52, Novosibirsk 630091
A. M. Volkov
Russian Federation
Alexander М. Volkov — Dr. Sci. (Med.), Leading Researcher, Laboratory of Experimental Surgery and Morphology, Institute of Experimental Biology and Medicine, Meshalkin National Medical Research Center, Ministry of Health of the Russian Federation.
Rechkunovskaya str., 15, Novosibirsk 600055
A. P. Nadeev
Russian Federation
Alexandr P. Nadeev — Dr. Sci. (Med.), Prof., Head of Anatomic Pathology Department, Novosibirsk State Medical University, Ministry of Health of the Russian Federation.
Krasny Prospect, 52, Novosibirsk 630091
A. V. Fomichev
Russian Federation
Alexei V. Fomichev — Cand. Sci. (Med.), cardiovascular surgeon, Cardiac Surgery Department No 2, Meshalkin National Medical Research Center, Ministry of Health of the Russian Federation.
Rechkunovskaya str., 15, Novosibirsk 600055
D. A. Sirota
Russian Federation
Dmitry A. Sirota — Cand. Sci. (Med.), Head of Research Department of Surgery on Aorta, Coronary and Peripheral Arteries, Institute of Circulatory Pathology, Meshalkin National Medical Research Center, Ministry of Health of the Russian Federation.
Rechkunovskaya str., 15, Novosibirsk 600055; Krasny Prospect, 52, Novosibirsk 630091
E. Е. Kliver
Russian Federation
Evgeniy Е. Kliver — Dr. Sci. (Med.), Head of Anatomic Pathology Department, Meshalkin National Medical Research Center, Ministry of Health of the Russian Federation; Prof., Anatomic Pathology Department, Novosibirsk State Medical University, Ministry of Health of the Russian Federation.
Rechkunovskaya str., 15, Novosibirsk 600055; Krasny Prospect, 52, Novosibirsk 630091
M. О. Zhulkov
Russian Federation
Maksim О. Zhulkov — Cand. Sci. (Med.), Researcher, Research Department of Surgery on Aorta, Coronary and Peripheral Arteries, Institute of Circulatory Pathology, Meshalkin National Medical Research Center, Ministry of Health of the Russian Federation.
Rechkunovskaya str., 15, Novosibirsk 600055
S. V. Pozdnyakova
Russian Federation
Svetlana V. Pozdnyakova — Dr. Sci. (Med.), Assoc. Prof., Prof. at Anatomic Pathology Department, Novosibirsk State Medical University, Ministry of Health of the Russian Federation.
Krasny Prospect, 52, Novosibirsk 630091
Supplementary files
Review
For citations:
Kliver V.Е., Volkov A.M., Nadeev A.P., Fomichev A.V., Sirota D.A., Kliver E.Е., Zhulkov M.О., Pozdnyakova S.V. Morphological assessment of actin and desmin expression at different cold myocardial ischemia times: observational study. Kuban Scientific Medical Bulletin. 2024;31(1):15-26. https://doi.org/10.25207/1608-6228-2024-31-1-15-26
JATS XML








































