Scroll to:
Optimization of the surgical approach to treating hepatic cystic echinococcosis: A retrospective observational non-randomized study
https://doi.org/10.25207/1608-6228-2024-31-3-17-29
Abstract
Background. Hepatic echinococcosis poses a great threat to human health and life. In order to minimize the risk of disability and speed up the postoperative recovery process, it is necessary to make an informed choice of the surgical treatment approach to be used. However, it is still unclear when traditional surgical methods should be applied and when less traumatic, minimally invasive, ultrasound-guided interventions can be used. This is what prompted this study. The article presents the results of using different surgical methods to treat patients with hepatic echinococcal cysts.
Aim. To improve the outcomes of treating patients with hepatic cystic echinococcosis by optimizing the surgical approach using percutaneous minimally invasive and traditional surgical interventions.
Methods. A non-randomized comparative study was conducted at the Sklifosovsky Institute for Emergency Medicine of the Moscow Department of Health. The subjects were treated in 2010–2020, with a follow-up period of four years after surgical treatment. A total of 78 patients with hepatic echinococcal cysts aged 18 to 78 years were treated. At the preoperative stage, medical history was collected from all patients, including data on migration during life. The diagnosis of all patients was confirmed through laboratory tests, instrumental methods (computed tomography and ultrasound), and a morphological examination of surgical material. All patients were divided into four groups: three groups of patients treated using different percutaneous minimally invasive methods and a comparison group of patients who underwent traditional surgery. The systematization of the material and its primary mathematical processing were performed using Excel 2016 (Microsoft, USA). The obtained numerical data were processed via methods of mathematical statistics using IBM SPSS Statistics 26 Version (IBM, USA).
Results. In the sample distribution of the studied groups by gender, age, as well as the number of cysts and concomitant diseases, no statistically significant differences were identified; however, the study yielded several results indicating the advantages of using minimally invasive treatment for hepatic echinococcal cysts. In spite of the high likelihood of biliary fistulas and a suppurative residual cavity occurring with the use of minimally invasive methods, a statistically significant reduction in the operation time, duration of anesthesia in the postoperative period, and blood loss were observed, which in turn reduced the stay in the inpatient surgical facility.
Conclusion. Minimally invasive methods can be used in all types of hepatic echinococcal cysts, as well as traditional surgery. It is reasonable to perform minimally invasive interventions if the clinic is adequately equipped and the surgeon is experienced in minimizing postoperative complications and recurrences.
For citations:
Boyarinov V.S., Rogal M.L., Novikov S.V., Dzhagraev K.R., Yartsev P.A. Optimization of the surgical approach to treating hepatic cystic echinococcosis: A retrospective observational non-randomized study. Kuban Scientific Medical Bulletin. 2024;31(3):17-29. https://doi.org/10.25207/1608-6228-2024-31-3-17-29
BACKGROUND
According to the World Health Organization (WHO), over two billion people worldwide suffer from hepatic echinococcosis. About 200 per 100 000 population are affected annually [1]. Cystic echinococcosis and alveolar echinococcosis are the two most common forms of human echinococcosis that are caused by the larval stages of Echinococcus granulosus and Echinococcus multilocularis, respectively [2][3].
In 2020, over 5000 confirmed cases of echinococcosis were reported in the European area. Of these, over half of these cases were caused by Echinococcus granulosus.1 The epidemiologic situation regarding the incidence of echinococcosis in Russia also remains difficult, with over 500 new cases of echinococcosis reported annually across the country2 [4]. Most commonly, echinococcal cysts occur in the liver (approximately 70% of all cases of echinococcosis) [5][6].
It is recommended to use chemotherapy for echinococcal cysts of under 5 cm. In case of negative cyst growth dynamics, minimally invasive treatment is possible [7][8].
The main strategy for surgical treatment of hepatic echinococcosis includes conventional echinococcectomy, as well as percutaneous minimally invasive interventions (PAI (puncture, aspiration, injection), PAIR (puncture, aspiration, injection, re-aspiration) and PEVAC (perifoveal exudative vascular anomalous complex)) [9][10]. Complications and risks after PAI and PAIR do not exceed those after traditional surgery [11].
Postoperative mortality is reported to be as high as 0.5–8%, and the rate of postoperative complications ranges from 12% to 80% depending on the type of surgical intervention, as well as the type and localization of echinococcal cysts. After open and minimally invasive surgical treatment, recurrence occurs within a wide range of 2%–22%3 [12][13].
This may be attributed to the absence of a single universally accepted protocol in the surgical treatment of hepatic echinococcosis that takes into account the form and morphological stage of the disease, which is due to the insufficient evidence to establish indications for choosing a surgical access (minimally invasive and traditional surgery), as well as methods for eliminating and treating residual cavity after echinococcectomy. Although percutaneous methods for removing hepatic echinococcal cysts under diagnostic imaging control are aimed at minimal trauma and rapid rehabilitation with the patient returning to everyday life and work in the shortest possible time, the number of clinics where percutaneous surgical interventions are used to treat echinococcal cysts is small in Russia [14][15].
In this connection, it is relevant to determine the superiority of one of the surgical methods (traditional or minimally invasive) for treating hepatic echinococcosis.
The study aims to improve the outcomes of treating patients with hepatic cystic echinococcosis by optimizing the surgical approach using percutaneous minimally invasive and traditional surgical interventions.
METHODS
Study design
A retrospective observational clinical study of patients with hepatic echinococcal cysts was conducted; seventy-eight patients participated in the study.
Eligibility criteria
Inclusion criteria
Patients with hepatic echinococcal cysts and patients with coexisting damage of liver and other parenchymal organs at various stages of life; inpatients treated at the Department for Emergency Surgery, Endoscopy, and Intensive Care (Surgical Department No. 2) of the Sklifosovsky Institute for Emergency Medicine in 2010–2020; patients aged from 18 to 78 years; size of echinococcal cysts from 5 cm and larger; patient’s consent for surgical intervention.
Exclusion criteria
Size of echinococcal cysts of under 5 cm.
Removal criteria
A patient’s refusal to further participate in the study, acute inflammatory diseases, and blood-clotting disorders.
Study conditions
The patients were treated in the Surgical Department No. 2 of the Sklifosovsky Institute for Emergency Medicine of the Moscow Department of Health in 2010–2020.
Study duration
The groups of subjects were selected and the results were recorded in 2010–2020. The observation period lasted ten years, while the follow-up period after surgical treatment was four years.
Description of the medical intervention
Clinical diagnosis was made on the basis of collected complaints, medical history, serologic testing, an abdominal ultrasound, and an abdominal CT scan, as well as cytologic or histologic examination of surgical specimens.
The leading modern methods for treating echinococcal disease are percutaneous minimally invasive interventions such as PAI (puncture, aspiration, injection), PAIR (puncture, aspiration, injection, re-aspiration), and PDE (percutaneous drainage, evacuation). In order to achieve the anti-scolecidal effect, the following germicides were used: glycerol (99%), sodium hypochlorite (1%), sodium chloride (30%), and ethanol (≥90%) [16]. Prior to choosing any surgical treatment option, irrespective of whether it is traditional or minimally invasive surgery, the surgical approach and possible complications were discussed with the patients.
In this study, the PDE under ultrasound and radiographic guidance was the predominant procedure among minimally invasive interventions. A total of 27 patients with cyst size greater than 5 cm underwent the PDE; the interventions were performed in patients with all types of cysts according to the Gharbi ultrasound classification [17]. All operations were performed in a specialized operating room equipped with Logiq e (China) and Esaote My lab 70, 500 (Italy) ultrasound systems having linear and 3.5–5 mHz curvilinear transducers, as well as C-arm systems OEC Elite (GE OEC Medical Systems, USA) and Arcadis Avantic. The surgical and anesthesia teams used lead aprons and collars (RA623 and RA614) to protect themselves from radiation exposure. All operations were performed under intravenous or endotracheal anesthesia with the patient lying on their back or side. Following a triple pre-operative skin treatment with an antiseptic solution, a safe trajectory was determined under ultrasound guidance, so that the liver parenchyma layer over the cyst was at least 5 cm and no large blood vessels, bile ducts, and pleural sinus were present on the needle path. Primary drainages were performed by means of a single-stage system using Balton pig-tail catheters with stylets (6Fr, 9Fr, and 12Fr); in all the cases, a “free-hand” procedure was applied. After a 3–5 mm skin incision under ultrasound guidance, a catheter was inserted into the cyst in a swift, instantaneous motion. Then, the mandrel with the stylet was removed from the system, and the tube was lowered into the cyst cavity to completely aspirate the contents under ultrasound guidance. The contents of the cyst cavity were submitted to the laboratory for cytologic examination and bacterial culture. The volume of aspirated fluid ranged from 30 mL to 3000 mL. In 76% of cases, clear, transparent contents with hydatid sand were noted, while 24% of cases exhibited turbid discharge with signs of infection. In order to timely detect communication with bile ducts, the contents of the cysts were analyzed for bile acids using a Total Bile Acid (TBA) assay kit [18]. Cystography was performed, with Ultravist 100 (Bayer ScheringPharma) used as the contrast agent. After excluding biliary fistulas, 99% glycerol was injected into the cyst cavity in the amount equal to 1/3–1/2 of the initially aspirated volume followed by exposure for ten minutes. After exposure, all glycerol was aspirated from the cyst cavity. A J-shaped guide or several Amplatz guides (COOK) were inserted into the cavity through the tube. Once the guides were inserted, the initial tubes were withdrawn. One of the guides, depending on the clinical situation, was used to widen the drainage channel with 18 Fr to 30 Fr bougies (Balton; COOK). After that, one guide wire was used to insert one Balton 9 Fr pig-tail tube and the second guide wire was used to insert a Balton silicone tube (from 18Fr to 30Fr depending on the cyst size). Once the tubes were replaced with those having a larger diameter, it became possible to evacuate chitinous shells from the cyst cavity. All chitinous shells were sent for a histologic examination. Tubes were fixed to the skin with two threads; urine collectors were connected to the drains; an aseptic dressing was applied to the wound. After surgery, the patients were transferred to the general surgery department. From the first hours of the postoperative period, a single dose of analgesia (a 2.0 ketoprofen solution) was administered intramuscularly; no physical limitations were present; the patients were mobilized in the first hours after surgery. At day 1 after surgery, a pleural cavity ultrasound, an abdominal ultrasound, and a fistulography were performed. With adequate drainage and aspiration and no fluid accumulations in the abdominal cavity and pleural cavities, the patients were discharged with functioning drainage tubes. Prior to discharge, the patients were instructed on the rules of cavity flushing through tubes, applying dressings, and how to act in unforeseen situations. At the outpatient treatment stage, it was recommended to flush trough tubes with 0.05% chlorhexidine aqueous solution diluted 1/2 with a 0.9% sodium chloride solution twice a day; the amount of flushing was discussed individually, taking into account the amount of initial drainage from the cyst. After surgery, the patients had a follow-up every 7–14–30 days, depending on the size of the primary cyst, anatomical features, and dynamics of residual cavity obliteration. As the amount of drainage through the tubes and the volume of the residual cavity decreased, the tubes were replaced with those having a smaller diameter. The tubes were replaced on an ambulatory basis under fluoroscopic guidance without anesthesia, taking the already-formed drainage channels into account. The time required to reduce the size of the residual cavity varied from 15 to 60 days. This method is not recommended in patients with the calcification of cyst walls due to prolonged or impossible obliteration of the residual cavity and elimination of the infectious process.
The PAIR method was used to treat 24 patients; all surgeries were performed according to the procedure previously described for the PDE.
Drainages were performed by means of a single-stage system using Balton pig-tail catheters with stylets: 6Fr or 9Fr depending on the anti-scolecidal agent to be administered. Sodium hypochlorite, sodium chloride, or ethanol were administered using 6 Fr catheters, while 9 Fr catheters were used for glycerol. In all the cases, a “free-hand” procedure was applied. After performing a fistulogram and excluding biliary fistulas, the cyst cavity was injected with glycerol (99%), sodium hypochlorite (1%), sodium chloride (30%), or ethanol (≥90%) in the amount equal to 1/3–1/2 of the initially aspirated volume, followed by exposure for ten minutes. After exposure, all germicide was aspirated from the cyst cavity and the tube was withdrawn.
After surgery, the patients were transferred to the general surgery department, and they required no analgesic therapy; At day 1, the patients were discharged to continue outpatient treatment.
After discharge, patients underwent an ultrasound examination after one month and then every three months after that.
This method was used in patients with all types of cysts.
The PAI method was used to treat 14 patients; all surgeries were performed according to the procedure previously described for the PDE and PAIR.
The puncture was performed under ultrasound guidance in a single-stage manner using a Chiba needle 18G. Following aspiration, the cyst cavity was injected with sodium hypochlorite (1%), sodium chloride (30%), or ethanol (≥90%) in the amount equal to 1/3–1/2 of the initially aspirated volume. Next, the puncture needle was removed. In the absence of adverse reactions after surgery, the patients were discharged at day 1.
In this study, the traditional surgery method is represented by pericystectomy with cold argon plasma electrocoagulation of the fibrous capsule; thirteen patients underwent the operation. All operations were performed under endotracheal anesthesia, with access to the cyst obtained by performing a J-shaped laparotomy. Then, intraoperative ultrasound was performed to determine the localization of cysts, to identify previously undetected cysts, and to determine how the cysts were located relative to the bile ducts and liver vessels. Prior to cyst isolation, the free abdominal cavity was carefully separated, first with dry tissues and then with tissues moistened with germicide; after that, the liver was mobilized using the traditional method, depending on the localization of the cyst. In order to prevent the dissemination of scolices in open surgeries, a special vacuum aspirator was used with a wide tip (3 cm and wider) having a capacity of 1000 mm of water and higher; this device enabled the cyst contents together with the chitinous shell to be promptly aspirated, completely excluding their leakage into the abdominal cavity. Next, the cyst was incised at the part of the capsule wall most protruding from the liver parenchyma. The cyst contents were aspirated; through the same incision, the entire cyst was filled with an 80–100% glycerin solution, with an exposure time of at least 10–15 minutes. The exposure time was crucial in ensuring that all the parasitic elements were killed.
Upon completing the antiparasitic treatment, the hole in the cyst was enlarged and the germicide and the remaining viable elements of echinococcal cysts were removed. Gloves and instruments were changed; cyst walls were incised; the inner surface of the fibrous capsule was carefully examined for biliary fistulas. After that, cold argon plasma electrocoagulation of the fibrous capsule was performed using a Covidien Argon Gas Delivery Unit II (USA). If after coagulation, no bile leakage was observed and hemostasis was adequate, the operation was completed with the drainage of the residual cavity using a 12 Fr pig-tail drainage tube or a silicone tube (from 18 Fr to 24 Fr) and layered closure of the wound. In the postoperative period, the patients were observed according to the generally accepted surgical approach. Chemotherapy was not administered to any of the operated patients.
Study outcomes
Main study outcome
It is planned to reduce the stay in the inpatient facility (inpatient days) and minimize the traumatic nature of surgical operations in patients with hepatic echinococcal cysts by using minimally invasive interventions.
Additional study outcomes
No additional outcomes are intended.
Subgroup analysis
Ninety-one patients were examined with only 78 patients included in the study. These patients underwent surgical interventions. Of the 13 patients excluded from the study, the size of the cysts was under 5 cm in four cases, pulmonary echinococcal cysts were detected in four cases, and five patients refused to undergo the surgery.
All patients included in the study were divided into four groups: three main groups of patients (PDE n = 27; PAI n = 14; PAIR n = 24) and the comparison group (n = 13). In the main groups, the patients were treated using percutaneous minimally invasive methods, while in the comparison group, the patients underwent traditional surgery.
The results obtained for the four groups were comparatively analyzed.
Methods for recording outcomes
The long-term treatment efficacy and the quality of life of patients with hepatic echinococcal cysts were assessed using the SF-36 questionnaire (prior to surgery and 12 months after surgery).
The MOSSF-36 questionnaire consists of 36 questions that cover eight health scales using scores of 0 to 100 to reflect a person’s degree of satisfaction and overall well-being.
The following parameters are considered: physical functioning (PF), role-physical (RP), bodily pain (BP), and general health (GH) scales comprising the physical component summary (PCS) score, as well as the vitality (VIT), social functioning (SF), role-emotional (RE), and mental health (MH) scales comprise the mental component summary (MCS) score. A higher score indicates a better quality of life. In case of complete health satisfaction, the maximum score is 100 points.
Statistical analysis
Principles behind sample size determination
The sample size was not determined in advance.
Statistical methods
The distribution analysis of quantitative indicators served as the basis for assessing the normality of distribution (Kolmogorov–Smirnov test) for the subsequent application of parametric or nonparametric methods. If the sample distribution followed the normal distribution of source data, descriptive statistics were applied in the form of arithmetic mean and standard deviation (M ± SD), while the comparative analysis was performed using parametric methods (Student’s t-test and F-test). If sample distributions did not follow the normal distribution of source data, descriptive statistics were applied in the form of the median and first (Q1 — 25%) and third quartiles (Q3 — 75%) — Me (Q1; Q3), while the comparative analysis was performed using nonparametric methods (Mann— Whitney U-test and Kruskal–Wallis test). For analysis of qualitative indicators in the form of proportions(percentages), four- or multi-way contingency tables (Pearson’s chi-squared test and Fisher’s exact test) were used. A p-value of ≤0.05 was considered statistically significant. The obtained data were statistically processed using Excel 2016 (Microsoft, USA). The obtained numerical data were processed via methods of mathematical statistics using IBM SPSS Statistics 26 Version (IBM, USA). The sample consisted of 91 patients; thirteen patients were excluded from the study and 78 patients were divided into four groups for analysis: 27 patients in the PDE group, 14 patients the in PAI group, 24 patients in the PAIR group, and 13 patients in the comparison group.
RESULTS
Study participants
The sample consisted of 91 patients: 13 patients were excluded from the study and 78 patients were divided into four groups for analysis.
Patients from the first three groups were treated using minimally invasive surgical methods (27 patients in the PDE group, 14 patients the in PAI group, and 24 patients in the PAIR group); in the comparison group, 13 patients underwent traditional surgery—pericystectomy with cold argon plasma electrocoagulation of the fibrous capsule. The block diagram of the study design is presented in Fig. 1.
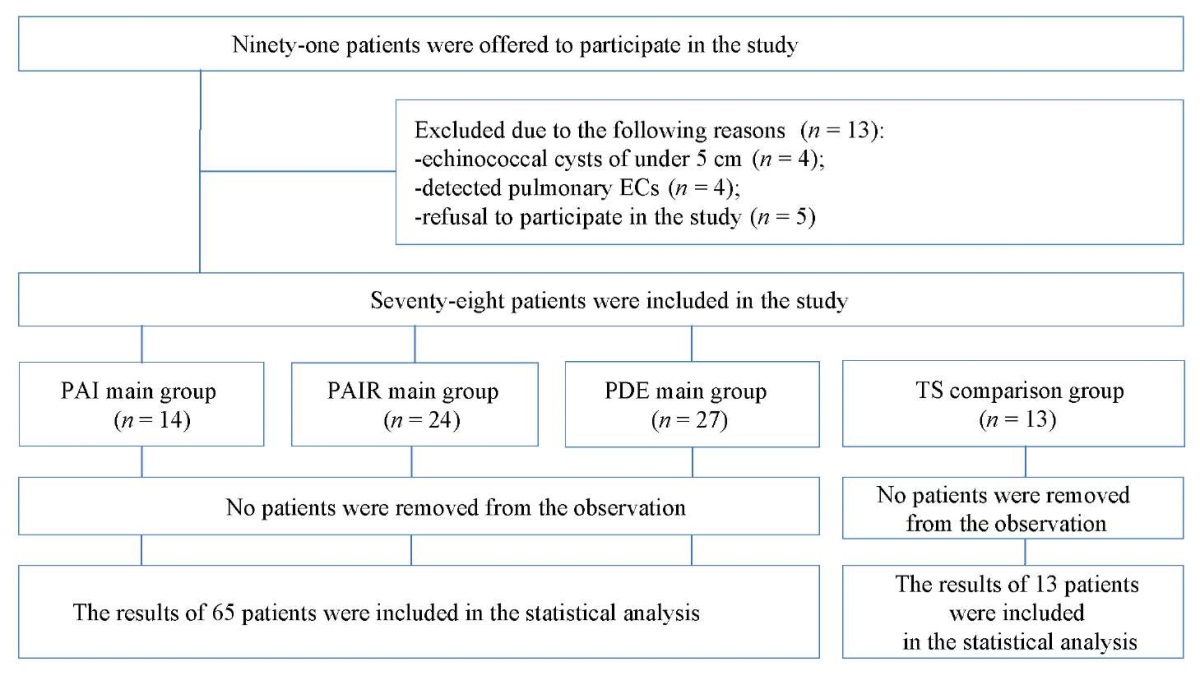
Fig. 1. Block diagram of the study design
Note: the block diagram was created by the authors (as per TREND recommendations). Abbreviations: EC — echinococcal cysts; PAI — puncture, aspiration, injection; PAIR– puncture, aspiration, injection, re-aspiration; PDE — percutaneous drainage, evacuation; TS — traditional surgery.
Рис. 1. Блок-схема дизайна исследования
Примечание: блок-схема выполнена авторами (согласно рекомендациям, TREND). Сокращения: EC — эхинококковые кисты; PAI — puncture, aspiration, injection (пункция, аспирация, введение гермицида); PAIR — puncture, aspiration, injection, re-aspiration (пункция, аспирация, введение гермицида, реаспирация); PDE — percutaneous drainage, evacuation (чрескожное дренирование, эвакуация); TS — традиционное хирургическое вмешательство.
Table 1 presents the general characterization of the four study groups in terms of complaints.
Table 1. Complaints in patients with hepatic echinococcal cysts
Таблица 1. Жалобы при эхинококковых кистах печени
|
Complaints |
Number of patients (abs. (%)) |
|
Asthenic syndrome |
6 (7.7%) |
|
Upper abdominal pain |
9 (11.4%) |
|
Decreased appetite |
2 (2.6%) |
|
Hyperthermia |
2 (2.6%) |
|
Allergic reaction |
2 (2.6%) |
|
Asymptomatic course |
57 (73.1%) |
|
Total |
78(100%) |
Note: the table was compiled by the authors.
Примечание: таблица составлена авторами.
The asymptomatic (latent) stage of echinococcosis was found in 57 (73.1%) patients, i.e., parasitic cysts were identified incidentally during abdominal ultrasound, while 21 (26.9%) patients exhibited symptoms. In the total sample, the following clinical symptoms were observed: epigastric and right upper quadrant abdominal pain (11.4%), cachexia (7.7%), allergic reactions (2.6%), decreased appetite (2.6%), and hyperthermia (2.6%). Statistically significant differences (according to the chi-squared test) were observed in the proportions of the three groups of patients with complaints. The patients with no symptoms formed the largest proportion (73.1%), followed by the patients with complaints about upper abdominal pain (11.4%) and the group of patients with other
complaints (15.5%), whose proportions were not statistically significantly different from each other (p > 0.05).
In order to establish sample homogeneity in the compared groups, several indicators were analyzed: gender composition of the groups; age; BMI of patients in the groups; number of patients with one cyst or with two or more cysts; number of patients with one concomitant disease or with two or more concomitant diseases. The specified indicators are given in Table 2.
Table 2. Values of some indicators in the compared patient groups
Таблица 2. Значения некоторых показателей сравниваемых групп пациентов
|
Indicator |
PDE group (n = 27) |
PAI group (n = 14) |
PAIR group (n = 24) |
Comparison group (n = 13) |
Significance level, p |
|
Gender (male/female) |
12/15 |
6/8 |
10/14 |
7/6 |
0.909* |
|
Age (М ± SD), (years) |
41.7 ± 15.6 |
44.9 ± 17.3 |
37.1 ± 13.9 |
38.0 ± 14.2 |
0.843# |
|
BMI (М ± SD), conventional units |
25.5 ± 3.2 |
25.8 ± 3.4 |
24.6 ± 4.6 |
24.4 ± 3.4 |
0.674# |
|
Number of patients with one/ more than one concomitant disease |
21/6 |
10/4 |
16/8 |
9/4 |
0.989* |
|
Number of patients having one / more than one cyst |
18/9 |
7/7 |
19/5 |
8/5 |
0.181* |
Note: the table was compiled by the authors; * — as per the chi-squared test, # — as per the F-test. Abbreviations: PAI — puncture, aspiration, injection; PAIR — puncture, aspiration, injection, re-aspiration; PDE — percutaneous drainage, evacuation; BMI — body mass index.
Примечания: таблица составлена авторами; * — по критерию Хи-квадрат, # — по F-критерию Фишера. Сокращения: PAI — puncture, aspiration, injection (пункция, аспирация, введение гермицида); PAIR — puncture, aspiration, injection, re-aspiration (пункция, аспирация, введение гермицида, реаспирация); PDE — percutaneous drainage, evacuation (чрескожное дренирование, эвакуация); BMI — индекс массы тела.
According to a multi-way contingency table, the gender composition of the groups was not statistically significantly different (p = 0.909): the lowest proportion of males (41.7%) was observed in the PAIR group, while the highest proportion (53.8%) was noted in the comparison group.
The proportions of patients with one concomitant disease were also not statistically significantly different between the study groups (p = 0.989) according to the chi-squared test: the lowest proportion (66.7%) was observed in the PAIR group and the highest proportion (77.8%), in the PDE group.
Similarly, the proportions of patients with a single cyst were also not statistically significantly different between the study groups (p = 0.181) according to the chi-squared test: the lowest proportion (50.0%) was observed in the PAI group, and the highest proportion (79.2%), in the PAIR group.
The preliminarily conducted distribution analysis of quantitative age and BMI (body mass index) values according to the Kolmogorov-Smirnov tests revealed that the distributions follow the normal distribution (p > 0.05). Therefore, analysis of variance F-test was used to compare the mean age and BMI in the groups (Table 2).
No statistically significant age difference between groups was found (p = 0.843): the lowest mean age of (37.1 ± 13.9) years was observed in the PAIR group, while the highest mean age of (44.9 ± 17.3) years was noted in the PAI group.
No statistically significant difference was seen among the groups with respect to the mean BMI (p = 0.674): the lowest mean BMI of (24.4 ± 3.4) was found in the comparison group, while the highest mean BMI of (25.8 ± 3.4) was observed in the PAI group.
According to all compared indicators, the differences between the groups were not statistically significant (p > 0.05), which indicates their homogeneity.
Main study results
Table 3 shows the medians of the SF-36 questionnaire scales prior to surgical treatment for hepatic echinococcal cysts. The analysis of medians revealed that all indicators—PCS (physical functioning, role-physical, bodily pain, and general health) and MCS (vitality, social functioning, role-emotional, and mental health)—are at the level of a healthy person, except for changes associated with age peculiarities and the presence of chronic disease. This is yet another example showing that hepatic echinococcosis is usually virtually asymptomatic.
Table 3. MOSSF 36 quality-of-life parameters (score) in patients
prior to surgery for hepatic echinococcal cysts
Таблица 3. Параметры качества жизни MOSSF-36 (в баллах) у пациентов
до хирургического вмешательства на эхинококковых кистах печени
|
MOSSF 36 scales (scores) |
PDE group (n = 27) |
PAI group (n = 14) |
PAIR group (n = 24) |
Comparison group (n = 13) |
|
PF |
90 |
95 |
90 |
90 |
|
RP |
90 |
85 |
80 |
90 |
|
BP |
95 |
95 |
95 |
90 |
|
GH |
80 |
90 |
85 |
85 |
|
VIT |
85 |
80 |
90 |
90 |
|
SF |
90 |
95 |
90 |
95 |
|
RE |
90 |
90 |
95 |
95 |
|
MH |
85 |
80 |
80 |
78 |
|
PCS |
55.1 |
55.7 |
53.5 |
53.7 |
|
MCS |
55.4 |
53.9 |
54.3 |
52.5 |
Note: the table was compiled by the authors; Abbreviations: MOSSF-36 — Medical Outcomes Study Short-Form 36; PAI — puncture, aspiration, injection; PAIR– puncture, aspiration, injection, re-aspiration; PDE — percutaneous drainage, evacuation; PF — physical functioning; RP — role-physical; BP — bodily pain; GH — general health; PCS — physical component summary; VIT — vitality; SF — social functioning; RE — role-emotional; MH — mental health; MCS — mental component summary.
Примечание: таблица составлена авторами; Сокращения: MOSSF-36 — Medical Outcomes Study Short-Form 36; PAI — puncture, aspiration, injection (пункция, аспирация, введение гермицида); PAIR– puncture, aspiration, injection, re-aspiration (пункция, аспирация, введение гермицида, реаспирация); PDE — percutaneous drainage, evacuation (чрескожное дренирование, эвакуация); PF — физическое функционирование; RP — ролевое физическое функционирование; BP — физическая боль; GH — общее состояние здоровья; PCS — физический компонент здоровья; VIT — жизненная активность; SE — социальное функционирование; RE — ролевое эмоциональное функционирование; MH — психологическое здоровье; MCS — психический компонент здоровья.
In the median analysis of the physical functioning, role-physical, bodily pain, and general health scales comprising the PCS score, 12 months after surgical treatment, it was found that the medians in the comparison group were lower as compared to the medians in the main group (PAI, PAIR, and PDE). The lower medians of the physical functioning and role-physical scales indicate that patients in the comparison group showed more restricted physical activity than those in the main group.
Of note is that the medians of the bodily pain scale characterizing pain intensity were lower in the comparison group, which reflects the more traumatic nature of surgical access in traditional interventions.
An analysis of the obtained data (Table 4) revealed that one year after minimally invasive treatment, the medians of MCS statistically increased in all patients as compared to the preoperative values. The results obtained for the MCS scales reached the level of practically healthy individuals.
Table 4. MOSSF 36 quality-of-life parameters (scores) in patients
12 months after surgery for hepatic echinococcal cysts
Таблица 4. Параметры качества жизни MOSSF-36 (в баллах) у пациентов
через 12 месяцев после хирургического вмешательства на эхинококковых кистах печени
|
MOSSF 36 scales (scores) |
PDE group (n = 27) |
PAI group (n = 14) |
PAIR group (n = 24) |
Comparison group (n = 13) |
|
PF |
95 |
100 |
92 |
80 |
|
RP |
95 |
95 |
95 |
85 |
|
BP |
100 |
100 |
100 |
90 |
|
GH |
90 |
95 |
95 |
90 |
|
VIT |
95 |
90 |
95 |
85 |
|
SF |
95 |
100 |
95 |
90 |
|
RE |
95 |
100 |
95 |
90 |
|
MH |
92 |
90 |
85 |
80 |
|
PCS |
57.3 |
58.5 |
57.8 |
52.1 |
|
MCS |
59.6 |
61.4 |
60.1 |
50.2 |
Note: the table was compiled by the authors; Abbreviations: MOSSF-36 — Medical Outcomes Study Short-Form 36; PAI — puncture, aspiration, injection; PAIR — puncture, aspiration, injection, re-aspiration; PDE — percutaneous drainage, evacuation; PF — physical functioning, RP — role-physical; BP — bodily pain; GH — general health; PCS — physical component summary; VIT — vitality; SF — social functioning; RE — role-emotional; MH — mental health; MCS — mental component summary.
Примечание: таблица составлена авторами; Сокращения: MOSSF-36 — Medical Outcomes Study Short-Form 36; PAI — puncture, aspiration, injection (пункция, аспирация, введение гермицида); PAIR– puncture, aspiration, injection, re-aspiration (пункция, аспирация, введение гермицида, реаспирация); PDE — percutaneous drainage, evacuation (чрескожное дренирование, эвакуация); PF — физическое функционирование; RP — ролевое физическое функционирование; BP — физическая боль; GH — общее состояние здоровья; PCS — физический компонент здоровья; VIT — жизненная активность; SE — социальное функционирование; RE — ролевое эмоциональное функционирование; MH — психологическое здоровье; MCS — психический компонент здоровья.
Several study findings are outlined, including operation time, duration of postoperative analgesia, length of stay in the intensive care unit, postoperative complications, length of hospitalization, and intraoperative blood loss.
A preliminary distribution analysis of quantitative indicators according to the Kolmogorov-Smirnov tests showed that some distributions follow the normal distribution (p > 0.05) (e.g., the number of inpatient days in the PDE group and in the PAIR group), while for some distributions of other indicators and in the other groups the hypothesis of normality was rejected (p < 0.05). Therefore, the indicators are presented in the form of medians and quartiles, and the comparison was performed using the Kruskal–Wallis test (Table 5).
Table 5. Values of some indicators (Me (Q1; Q3))
following surgical treatment in the compared groups
Таблица 5. Значения некоторых показателей (Ме (Q1; Q3))
по результатам хирургического лечения в сравниваемых группах
|
Indicators |
PDE group (n = 27) |
PAI group (n = 14) |
PAIR group (n = 24) |
Comparison group (n = 13) |
Significance level, p |
|
Operation time (min) |
15 (15;16) |
7 (6;9) |
13 (12;13) |
310 (290;330) |
р < 0.001 |
|
Duration of postoperative analgesia (days) |
1 (1;1) |
0 (0;0) |
0 (0;0) |
3 (3;3) |
р = 0.012 |
|
Stay in the intensive care unit (inpatient days) |
0 (0;0) |
0 (0;0) |
0 (0;0) |
1 (1;2) |
р = 0.006 |
|
Intraoperative blood loss, mL |
10 (8;10) |
4 (3;5) |
4 (4;5) |
150 (150;200) |
р < 0.001 |
|
Stay in the inpatient facility (inpatient days) |
19 (15;25) |
3 (2;5) |
15 (10;27) |
25 (17;39) |
р = 0.045 |
Note: the table was compiled by the authors; Abbreviations: PAI — puncture, aspiration, injection; PAIR — puncture, aspiration, injection, re-aspiration; PDE — percutaneous drainage, evacuation.
Примечание: таблица составлена авторами; Сокращения: PAI — puncture, aspiration, injection (пункция, аспирация, введение гермицида); PAIR — puncture, aspiration, injection, re-aspiration (пункция, аспирация, введение гермицида, реаспирация); PDE — percutaneous drainage, evacuation (чрескожное дренирование, эвакуация).
The median operation time in the studied groups ranged from 7 min in the PAI group, 13 min in the PAIR group, 15 min in the PDE group, and to 310 min in the comparison group, which are statistically significantly different (p < 0.001). The pairwise Mann-Whitney test also showed a significant difference between the median values of the PAI group and the other two main groups, PAIR and PDE. No significant difference was found between the medians of the PAIR and PDE groups (p > 0.05). However, for all the groups, the operation time was significantly shorter as compared to the comparison group: for example, by 20.7 times for the PDE group, and an even greater difference was observed for the other groups.
The median duration of postoperative analgesia in the PDE group was 1 (1;1) day, whereas in the comparison group, it amounted to 3 (3;3) days (p = 0.012). In the PAI and PAIR groups, this indicator was equal to zero (Table 5).
The indicator “stay in the intensive care unit (inpatient days)” was not observed for the PDE, PAI, and PAIR groups, while in the comparison group, the median was 1 (1;2) day (p = 0.006).
The length of hospitalization (inpatient days) in all groups was statistically significantly different according to the Kruskal–Wallis test (p = 0.045). A pairwise Mann—Whitney test showed the median value to be significantly greater in the comparison group (25 (17;19) days) than in all the other groups (p < 0.05): 19 (15;25) days for the PDE group, 3 (2;5) days for the PAI group, and 15 (10;27) days for the PAIR group (Table 5).
The extent of blood loss in all groups was statistically significantly different according to the Kruskal–Wallis test (p < 0.001). For the groups of patients who underwent minimally invasive innervations, blood losses were significantly lower than those in the comparison group (p < 0.001) according to the pairwise Mann-Whitney test. In the comparison group, the intraoperative blood loss amounted to 150 (150;200) mL. The same indicator was equal to 10 (8;10) mL in the PDE group, 4 (3;5) mL in the PAI group, and 4 (4;5) mL in the PAIR group, which in turn ensures early social and vocational rehabilitation of patients.
The proportion of postoperative complications in groups of patients who underwent minimally invasive innervations was 13.8% (9 complications out of 65 operations). In the comparison group, it amounted to 15.4% (2 complications out of 13 surgeries). No statistically significant difference was found according to the chi-squared test, p = 0.359 (Table 6).
Table 6. Number of postoperative complications in the compared groups
Таблица 6. Количество послеоперационных осложнений в группах сравнения
|
Indicators |
PDE group (n = 27) |
PAI group (n = 14) |
PAIR group (n = 24) |
Comparison group (n = 13) |
Significance level, p |
|
Biliary fistula |
2 |
0 |
4 |
2 |
|
|
Abdominal abscess |
0 |
0 |
0 |
0 |
|
|
Bleeding |
0 |
0 |
0 |
0 |
|
|
Suppurative residual cavity |
2 |
0 |
1 |
0 |
|
|
Postoperative wound suppuration |
0 |
0 |
0 |
0 |
|
|
Residual cavity |
0 |
0 |
0 |
0 |
|
|
Total |
4 |
0 |
5 |
2 |
0.359 |
Note: the table was compiled by the authors; Abbreviations: PAI — puncture, aspiration, injection; PAIR — puncture, aspiration, injection, re-aspiration; PDE — percutaneous drainage, evacuation.
Примечание: таблица составлена авторами; Сокращения: PAI — puncture, aspiration, injection (пункция, аспирация, введение гермицида); PAIR — puncture, aspiration, injection, re-aspiration (пункция, аспирация, введение гермицида, реаспирация); PDE — percutaneous drainage, evacuation (чрескожное дренирование, эвакуация).
This fact was attributed to the presence of patients with postoperative complications developed during treatment at other health facilities. Patients were often unable to even name the extent of the performed operation, provide medical documentation, and certainly had not heard of a postoperative follow-up.
One of the frequent complications was suppurative residual cavity. This complication was eliminated through flushing with antiseptic solutions or additional drainage. In all the cases, external biliary fistulas were eliminated in the complete obliteration of the residual cavity. The comprehensive treatment was supplemented with hymecromone, at a dose of 200 mg for an average of 14 days until the cessation of bile
secretion through the tube. No additional interventions were required. The study did not reveal such a complication as sclerosing cholangitis to the use of hypertonic saline, glycerol, and alcohol.
The most frequent complication in surgical practice is bleeding. The occurrence of this complication during percutaneous surgeries carries an additional risk since a conversion may be required in this case. Bleeding during minimally invasive interventions can occur either due to direct damage to large vessels or rupture of the liver capsule and parenchyma that occur while moving the instrument. In order to avoid such complications, it is necessary to choose the correct tool path and make sure that no vascular structures are in the way. It is important to see the needle throughout the operation, especially when it penetrates the liver parenchyma. In the study, no such complications were observed in any of the patients. In order to achieve definitive hemostasis during traditional interventions, we used argon plasma electrocoagulation methods or local hemostatic means (sponge; matrix) applied to the wound surface of the liver parenchyma (Table 4).
The same principles should be followed to prevent biliary duct damage. No damage to bile ducts during minimally invasive interventions was noted in any of the observations.
Allergic reactions of the body caused by parasitic invasion are characteristic signs of echinococcosis and are quite common. Since the products of echinococcosis are strong allergens, the leakage of cyst contents into the free abdominal cavity can lead to allergic reactions of varying severity. The risk is particularly high when the cyst is entered through the free edge of an extraparenchymal cyst or the liver parenchyma layer over the cyst at the puncture site is too thin. In such cases, hydatid fluid may leak along the puncture path. No allergic reactions occurred in all the operated patients.
Subsequently, all patients were observed for 4–5 years at the Sklifosovsky Institute for Emergency Medicine of the Moscow Department of Health, which included abdominal ultrasounds, abdominal CT scans, and serological blood tests. The recurrence rate of hepatic echinococcosis, as well as mortality, in all cases was 0%.
Additional study results
No additional results were obtained during the study.
Adverse events
No adverse events were observed during the study.
DISCUSSION
Summary of the main study result
The study showed the advantage of using minimally invasive methods for treating hepatic echinococcal cysts due to a shorter surgical intervention time and minimal intraoperative blood loss. All the patients from the main group did not require postoperative treatment in the intensive care unit. Due to the less traumatic nature of operations and earlier mobilization, the length of hospitalization is reduced (from one to two days). An important result of the conducted study consists in obtaining efficacy data according to the PCS and MCS scales. They indicate that one year after treatment, the efficacy of minimally invasive treatment in patients from the main group was superior to that in the comparison group.
Discussion of the main study result
If untreated, echinococcal cysts grow and develop in one of the following ways: spontaneous inactivation; rupture into the abdominal cavity with the spread of multiple daughter cysts throughout the abdominal cavity; development of fistulas to neighboring organs or the biliary system [19].
The main treatment options for hepatic cystic echinococcosis include the wait-and-see approach, drug therapy, traditional surgery, and the use of minimally invasive methods.
According to the WHO, surgery remains a cornerstone in the treatment of echinococcosis, as this method can eliminate the echinococcal cyst, resulting in complete recovery [20]. The most acute problem in treating patients with hepatic echinococcosis is the choice of a surgical method. Although liver surgery has advanced significantly, several issues remain unresolved regarding the use of minimally invasive methods for CE2, CE3a, and CE3b types of cysts. In this study, we used minimally invasive methods for all types of cysts despite certain limitations imposed by foreign and domestic authors.
The conducted study yielded several results indicating the advantages of using minimally invasive methods: increased efficacy, lower recurrence rates, and shorter stays in the inpatient facility, which in turn ensured early social and vocational rehabilitation of patients.
Despite the high probability of postoperative complications when using minimally invasive methods, a statistically significant reduction in the operation time and duration of anesthesia in the postoperative period was observed, which in turn led to a shorter stay in the surgical inpatient facility.
Given the lack of up-to-date national and foreign clinical guidelines, the question remains regarding the management of hepatic echinococcosis in patients, which makes this study relevant.
Research limitations
Not identified.
CONCLUSION
Minimally invasive ultrasound guided percutaneous surgical interventions for hepatic echinococcosis have been used along with other surgical methods since 1985. With the technological advancement of surgery and a trend toward less traumatic surgical treatment methods, the differentiated approach to choosing a method of surgical treatment in patients with hepatic cystic echinococcosis remains a relevant issue.
Such indicators as surgical intervention time, duration of analgesia in the postoperative period, length of stay in the intensive care unit, length of hospitalization, and intraoperative blood loss were statistically significantly lower in the groups of patients who underwent minimally invasive interventions than in the comparison group.
1.European Centre for Disease Prevention and Control. Surveillance systems overview. Stockholm: ECDC; 2021. Available: https://www.ecdc.europa.eu/en/publications-data/surveillance-systems-overview-2021
2. Demidenko LA, Fedorets AV, Namazova LE. Development dynamics of echinococcosis in Russia. Chelovek-Priroda-Obshchestvo: Teoriia i Praktika Bezopasnosti Zhiznedeiatel’nosti, Ekologii i Valeologii. 2019;5(12):136-140.
3. Ikramov RZ, Zhavoronkova OI, Botiraliev ASh, Olifir AA, Stepanova YuA, Vishnevskii VA, Zhao AV. Modern approaches in the treatment of hepatic echinococcosis. Vysokotekhnologicheskaya Meditsina. 2020;7(2):14-27.
References
1. Lee PC, Lo C, Lai PS, Chang JJ, Huang SJ, Lin MT, Lee PH. Randomized clinical trial of single-incision laparoscopic cholecystectomy versus minilaparoscopic cholecystectomy. Br J Surg. 2010;97(7):1007–1012. https://doi.org/10.1002/bjs.7087
2. Woolsey ID, Miller AL. Echinococcus granulosus sensu lato and Echinococcus multilocularis: A review. Res Vet Sci. 2021;135:517–522. https://doi.org/10.1016/j.rvsc.2020.11.010
3. Alvi MA, Ali RMA, Khan S, Saqib M, Qamar W, Li L, Fu BQ, Yan HB, Jia WZ. Past and present of diagnosis of echinococcosis: A review (1999–2021). Acta Trop. 2023;243:106925. https://doi.org/10.1016/j.actatropica.2023.106925
4. Dragomeretskaya AG, Trotsenko OE, Logvin FV, Tverdokhlebova TI, Romanova EB, Ishchenkova IV, Moskvina YuI, Dimidova LL, Chernikova MP. The current situation of echinococcosis in the Far East and South of Russia. Medical Herald of the South of Russia. 2024;15(1):27–35 (In Russ.). https://doi.org/10.21886/2219-8075-2024-15-1-27-35
5. Skvortsov VV, Levitan BN, Gorbac AN. Echinococcosis and Other Parasitic Liver Diseases. Effective Pharmacotherapy. 2020;16(30):88–91 (In Russ.). https://doi.org/10.33978/2307-3586-2020-16-30-88-91
6. Baruah A, Sarma K, Barman B, Phukan P, Nath C, Boruah P, Rajkhowa P, Baruah M, Dutta A, Naku N. Clinical and Laboratory Presentation of Hydatid Disease: A Study From Northeast India. Cureus. 2020;12(9):e10260. https://doi.org/10.7759/cureus.10260
7. Aliev MZh, Niyazbekov KI. Efficiency of conservative treatment of echinococcosis. Vestnik Smolenskoj Gosudarstvennoj Medicinskoj Akademii. 2020;19(4):117–22 (In Russ.). https://doi.org/10.37903/vsgma.2020.4.19
8. Salm LA, Lachenmayer A, Perrodin SF, Candinas D, Beldi G. Surgical treatment strategies for hepatic alveolar echinococcosis. Food Waterborne Parasitol. 2019;15:e00050. https://doi.org/10.1016/j.fawpar.2019.e00050
9. Wan L, Wang T, Cheng L, Yu Q. Laparoscopic Treatment Strategies for Liver Echinococcosis. Infect Dis Ther. 2022;11(4):1415–1426. https://doi.org/10.1007/s40121-022-00664-2
10. Öztürk G, Uzun MA, Özkan ÖF, Kayaalp C, Tatli F, Eren S, Aksungur N, Çoker A, Bostanci EB, Öter V, Kaya E, Taşar P. Turkish HPB Surgery Association consensus report on hepatic cystic Echinococcosis (HCE). Turk J Surg. 2022;38(2):101–120. https://doi.org/10.47717/turkjsurg.2022.5757
11. Efanov M, Azizzoda Z, Elizarova N, Alikhanov R, Karimkhon K, Melekhina O, Kulezneva Y, Kazakov I, Vankovich A, Chitadze A, Salimgereeva D, Tsvirkun V. Laparoscopic radical and conservative surgery for hydatid liver echinococcosis: PSM based comparative analysis of immediate and long-term outcomes. Surg Endosc. 2022;36(2):1224–1233. https://doi.org/10.1007/s00464-021-08391-4
12. Musaev GKh, Fat’ianova AS, Levkin VV. Principles and modern trends in liver echinococcosis treatment. Pirogov Russian Journal of Surgery. 2017;(12):90–94 (In Russ.). https://doi.org/10.17116/hirurgia20171290-94
13. Wang Z, Zhu HH, Yang JY, Wang Y, Gai ZG, Ma FC, Yang DW. Laparoscopic versus conventional open treatment of hepatic cystic hydatidosis: a systematic review and meta-analysis of cohort studies. Wideochir Inne Tech Maloinwazyjne. 2022 Sep;17(3):406–417. https://doi.org/10.5114/wiitm.2022.115225
14. Kirtanasov YaP, Ivshin VG. Percutaneous interventions in the treatment of patients with multi hydatid echinococcosis of the liver. Journal of New Medical Technologies. 2019;2:23–32 (In Russ.). https://doi.org/10.24411/2075-4094-2019-16365
15. Zarivchatsky MF, Mugattarov IN, Kamenskikh ED, Kolyvanova MV, Teplykh NS. Surgical treatment of liver echinococcosis. Perm Medical Journal. 2021;38(3):32–40 (In Russ.). https://doi.org/10.17816/pmj38332-40
16. Cai D, Li Y, Jiang Y, Wang H, Wang X, Song B. The role of contrast-enhanced ultrasound in the diagnosis of hepatic alveolar echinococcosis. Medicine (Baltimore). 2019;98(5):e14325. https://doi.org/10.1097/MD.0000000000014325
17. Liu W, Delabrousse É, Blagosklonov O, Wang J, Zeng H, Jiang Y, Wang J, Qin Y, Vuitton DA, Wen H. Innovation in hepatic alveolar echinococcosis imaging: best use of old tools, and necessary evaluation of new ones. Parasite. 2014;21:74. https://doi.org/10.1051/parasite/2014072
18. Khuroo MS. Percutaneous Drainage in Hepatic Hydatidosis-The PAIR Technique: Concept, Technique, and Results. J Clin Exp Hepatol. 2021 Sep-Oct;11(5):592–602. https://doi.org/10.1016/j.jceh.2021.05.005
19. Shamsiev AM, Shamsiev YuA, Rakhmanov KE, Dovlatov SS. Differentiated therapeutic tactics in surgery of liver echinococcosis. Experimental and Clinical Gastroenterology. 2020;5(177):72–77 (In Russ.). https://doi.org/10.31146/1682-8658-ecg-177-5-72-77
20. Mönnink GLE, Stijnis C, van Delden OM, Spijker R, Grobusch MP. Percutaneous Versus Surgical Interventions for Hepatic Cystic Echinococcosis: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2021;44(11):1689–1696. https://doi.org/10.1007/s00270-021-02911-4
About the Authors
V. S. BoyarinovRussian Federation
Vladimir S. Boyarinov — surgeon, Surgical Department
Bolshaya Sukharevskaya Sq., 3, Moscow, 129090
M. L. Rogal
Russian Federation
Mikhail L. Rogal — Dr. Sci. (Med.), Prof., Deputy Director for Science
Bolshaya Sukharevskaya Sq., 3, Moscow, 129090
S. V. Novikov
Russian Federation
Sergey V. Novikov — Dr. Sci. (Med.), Leading Researcher, Department for Emergency Surgery, Endoscopy, and Intensive Care
Bolshaya Sukharevskaya Sq., 3, Moscow, 129090
K. R. Dzhagraev
Russian Federation
Karen R. Dzhagraev — Cand. Sci. (Med.), Deputy Chief of Surgery
Bolshaya Sukharevskaya Sq., 3, Moscow, 129090
P. A. Yartsev
Russian Federation
Peter A. Yartsev — Dr. Sci. (Med.), Prof., Head of the Department for Emergency Surgery, Endoscopy, and Intensive Care
Bolshaya Sukharevskaya Sq., 3, Moscow, 129090
Supplementary files
Review
For citations:
Boyarinov V.S., Rogal M.L., Novikov S.V., Dzhagraev K.R., Yartsev P.A. Optimization of the surgical approach to treating hepatic cystic echinococcosis: A retrospective observational non-randomized study. Kuban Scientific Medical Bulletin. 2024;31(3):17-29. https://doi.org/10.25207/1608-6228-2024-31-3-17-29








































