Scroll to:
Geriatric factors affecting the safety of direct oral anticoagulants in atrial fibrillation patients aged 80 years and older: An observational prospective cohort study
https://doi.org/10.25207/1608-6228-2025-32-3-15-35
Abstract
Background. Older age is associated with the presence of geriatric syndromes, whose effect on the safety of direct oral anticoagulants in atrial fibrillation patients is studied extensively. Scientific literature focuses on the prevention of major bleeding, while clinically relevant non-major bleeding occurs much more frequently and can have a significant impact on the patient’s condition. Objective. To assess the potential of geriatric factors for predicting the risk of clinically relevant non-major bleeding on direct oral anticoagulants in nonvalvular atrial fibrillation patients aged 80 years and older. Methods. The article presents the results of an observational prospective cohort study that included 367 atrial fibrillation patients aged 80 years and older on direct oral anticoagulants. The study was conducted at a multi-specialty inpatient clinic in Moscow from January 2019 to December 2022 and reflected real clinical practice. The medical records were prospectively analyzed for the presence of clinically relevant non-major bleeding; the observation period lasted 12 months. The patients were divided into groups: the main group comprising patients with clinically relevant non-major bleeding (n = 195), with a median age of 84 [82; 87] years, and the control group consisting of patients without clinically relevant non-major bleeding (n = 172), with a median age of 84 [82; 88] years. The groups were comparable in terms of key bleeding risk factors. In order to identify geriatric risk factors, a comprehensive geriatric assessment was performed in four main domains: physical status; mental and emotional status; functional capacity; identification of social problems. Statistical data processing and visualization were performed in the R software environment, version 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria). Differences were considered statistically significant at p < 0.05. Results. The performed analysis identified risk factors for clinically relevant non-major bleeding in all four domains of the comprehensive geriatric assessment: social (living in a family, number of household members, legal marriage, and church attendance); mental and emotional (Geriatric Depression Scale); physical and functional (balance tests, Lawton IADL scale, use of assistive devices, senile asthenia as per the Age is No Barrier questionnaire, SPRINT (Systolic Blood Pressure Intervention Trial) frailty index, p < 0.05). In the course of a multiple-factor analysis, a multiple-factor logistic regression model was created to predict clinically relevant non-major bleeding, and a prognostic nomogram was derived to estimate the probability of clinically relevant non-major bleeding (prognostic accuracy of 59.9%, sensitivity of 48.9%, and specificity of 72.4%). The predictors were as follows: number of household members, Barthel ADL score, Lawton IADL score, Timed Up and Go Test, and use of any assistive device. Conclusion. In the study, socially active, emotionally stable, mobile, and robust patients were shown to have a higher risk of developing clinically relevant non-major bleeding events. The authors identified geriatric factors that may serve as predictors of clinically relevant non-major bleeding on direct oral anticoagulants in atrial fibrillation patients aged over 80 years. Given the active AI implementation in clinical practice, the obtained data can be integrated into clinical decision support systems.
Keywords
For citations:
Cherniaeva M.S., Pavlova A.O., Alferova P.A., Rozhkova M.A., Trifonov M.I., Nikeeva T.V., Egorova L.A., Maslennikova O.M., Lomakin N.V., Sychev D.A. Geriatric factors affecting the safety of direct oral anticoagulants in atrial fibrillation patients aged 80 years and older: An observational prospective cohort study. Kuban Scientific Medical Bulletin. 2025;32(3):15-35. https://doi.org/10.25207/1608-6228-2025-32-3-15-35
INTRODUCTION
Atrial fibrillation (AF) is a common condition that occurs in 1–2% of the general population, with the number of AF patients increasing with age, from <0.5% at ages 40–50 to 5–15% at the age of 80. The number of AF patients is expected to nearly double in the next 50 years. This pathology is associated with a high risk of systemic thromboembolism and ischemic stroke, which can be prevented via treatment with direct oral anticoagulants (DOACs) [1][2]. However, their use may cause bleeding, especially in elderly patients due to a number of age-related changes in the body. In particular, decreased hepatic perfusion and reduced activity of some cytochrome P450 isoforms affect the pharmacokinetics of DOACs, while the age-related decrease in the number of functioning nephrons increases the elimination half-life of these drugs. The receptor sensitivity and distribution density are modified, which directly affects the pharmacodynamic properties of DOACs1 [3–5].
Old age is known to be associated with the presence of geriatric syndromes, which include chronic pain syndrome, urinary/fecal incontinence, high fall risk, cognitive impairments, dementia, decreased mobility, frailty, etc. Frailty constitutes a major geriatric syndrome causing a decrease in the physiological reserves of the body.2
In the 2020 clinical guidelines of the European Society of Cardiology (ESC) on AF diagnosis and treatment [6], the HAS-BLED scale (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (> 65 years), drugs/alcohol concomitantly) assessed patient frailty, and risk factors for bleeding complications included certain geriatric syndromes, e.g., cognitive impairments or dementia and high fall risk. The ESC clinical guidelines for AF treatment revised in 2024 [2] abandon HAS-BLED bleeding risk assessment since systematic reviews and controlled trials in comparison groups showed highly inconsistent results and little prognostic value while focusing on the assessment and management of modifiable and potentially modifiable risk factors for bleeding, which include a high fall risk and cognitive impairments or dementia. The effect of frailty on the development of bleeding is mentioned neither in the 2024 EOC guidelines for AF treatment [2] nor in the clinical guidelines for the treatment of atrial fibrillation and flutter of the Russian Society of Cardiology [1].
Nevertheless, the results of studies in elderly AF patients receiving DOACs show that the risk of falls and frailty increase the risk of bleeding [7–11]. These studies primarily focus on major and intracranial bleeding, with clinically relevant non-major bleeding (CRNMB) not considered as a separate group. Nevertheless, the incidence of CRNMB is much higher than that of major bleeding, reaching 17.4% and increasing with age [12][13]. This complication should not be underestimated, as it is a reason for DOAC discontinuation, which increases the risk of thromboembolism and, consequently, disability or a fatal outcome. In addition, CRNMB can lead to major bleeding or patient hospitalization and a medical intervention [12]. We found no information on the contribution of other geriatric syndromes to bleeding events in patients receiving DOACs.
Current studies, therefore, lack data on geriatric syndromes as predictors of CRNMB on DOACs. Furthermore, the mean age of patients in studies examining the relationship between geriatric syndromes and DOAC safety ranges from 75 to 78 years, while the number of patients aged over 80 years is rising worldwide with increasing life expectancy [14][15]. This fact prompted the present study.
The present study aims to assess the potential of geriatric factors for predicting the risk of clinically relevant non-major bleeding on direct oral anticoagulants in nonvalvular atrial fibrillation patients aged 80 years and older.
METHODS
Study design
The observational prospective cohort study of actual clinical practice included 367 AF patients on DOACs aged ≥80 years who were treated at a multi-specialty inpatient clinic in Moscow from January 2019 to December 2022.
Fig. 1 Block diagram of the study design
Note: The block diagram was created by the authors (as per STROBE recommendations).
Рис. 1. Блок-схема дизайна исследования
Примечание: блок-схема выполнена авторами (согласно рекомендациям STROBE).
Study conditions
The study was conducted at the Department of Clinical Pharmacology and Therapy named after Academician B.E. Votchal, Russian Medical Academy of Continuous Professional Education, Ministry of Health of the Russian Federation. The patients were included in the study from January 2019 to December 2022. After that, outpatient and inpatient medical records were prospectively analyzed to determine the incidence of CRNMB during DOAC therapy. The observation period lasted one year.
Eligibility criteria
Inclusion criteria
Nonvalvular atrial fibrillation patients of both sexes; aged 80 years or older at study entry; regular DOAC use for at least one year from the time of study entry; signed voluntary informed consent for participation in the study.
Exclusion criteria
Age of <80 years; clinically significant heart disease (including cardiogenic shock; recent, i.e., less than one month prior to the study, complicated myocardial infarction; third-degree atrioventricular block without artificial pacemaker; hypertrophic cardiomyopathy; severe aortic and mitral stenosis); significant liver diseases (including liver cirrhosis with ascites); patients receiving renal replacement therapy (long-term hemodialysis; peritoneal dialysis; history of kidney transplant); clinically significant immunologic disease; neurological diseases (including acute cerebrovascular disorders and transient ischemic attack less than three months prior to the study); surgical procedure (except for dental or cosmetic surgeries), injuries, and fractures within the previous three months; presence of clinically significant changes in laboratory parameters indicating undiagnosed disease and requiring further examination.
Removal criteria
Violation of the examination and treatment procedures by the patient; refusal of the patient to participate in the study and withdrawal of informed consent.
Description of eligibility criteria (diagnostic criteria)
The diagnosis of AF was made according to the criteria of clinical guidelines for the treatment of atrial fibrillation and flutter of the Russian Society of Cardiology [1]. In patients receiving DOACs, CRNMB was diagnosed using criteria developed by the International Society on Thrombosis and Haemostasis (ISTH) [12]. The list of interventions performed on patients is regulated by the order of the Ministry of Health of the Russian Federation of January 29, 2016 (Order No. 38n) “On Approval of the Geriatric Medical Care Protocol” as amended on December 20, 2019 (Order No. 1067n).3
Selection of group members
The study cohort included 367 patients aged ≥80 years who were consecutively enrolled in the study, provided they met the inclusion criteria. At the end of the one-year observation, the patients were divided into two groups according to the data on CRNMB manifestation and recurrence: the main group included patients with CRNMB (n = 195), and the control group comprised patients without CRNMB (n = 172).
Target parameters in the study
Main parameters in the study
In order to identify geriatric factors affecting the development of CRNMB in patients on DOACs, patients underwent a comprehensive geriatric assessment, which included analysis in four main domains: physical status; mental and emotional status; functional capacity; identification of social problems.4
Additional parameters in the study
Additional parameters are not provided in this study.
Methods for measuring the target parameters
The study used the following validated charts, questionnaires, scales, tests, and estimated indices: a chart of comprehensive geriatric assessment,5 the Age is No Barrier questionnaire, self-assessment of quality of life and health status on the visual analogue scale (VAS), the Barthel Index for Activities of Daily Living (ADL) [16], the Lawton Instrumental Activities of Daily Living (Lawton IADL) Scale [17], Mini Nutritional Assessment [17][18], Short Physical Performance Battery, Timed Up and Go Test, and single-leg stance test.6 Also, all patients underwent dynamometry to determine handgrip strength; the criteria for low handgrip strength were established depending on sex and body mass index as per the clinical guidelines for frailty.2
The frailty index was calculated on the Rockwood scale [19] and on the scale used in the SPRINT (Systolic Blood Pressure Intervention Trial) study [20].
Mental and emotional status was assessed using the following tests, scales, and questionnaires7: Mini-Cog screening test, Mini-Mental State Examination, Montreal Cognitive Assessment Scale, Word List Recall, Digit Symbol Substitution Test, 10-word-list recall test, verbal fluency test (literal fluency/letters and category fluency/animals), Boston Naming Test, Functional Activities Questionnaire, Alzheimer’s Disease Assessment Scale-Cognitive, as well as Geriatric Depression Scale (GDS), GDS-15 (short form) and GDS-30 (long form).
Variables (predictors, confounders, and effect modifiers)
In the present study, factors affecting the final outcome were categorized as exclusion criteria, thus completely excluding the possibility of their presence in the examined patients.
Statistical procedures
Principles behind sample size determination
The sample size was not determined in advance.
Statistical methods
Statistical data analysis and visualization were performed in the R software environment, version 4.4.0 (R Foundation for Statistical Computing, Austria). Descriptive statistics are presented as absolute and relative frequencies for qualitative variables; as medians (Me) (Q1; Q3), for quantitative variables. Pearson’s χ² test and Fisher’s exact test were used to compare the groups in terms of categorical indicators (with a minimum expected number of observations <5 in the cells of the contingency table). The Mann—Whitney test was used to compare the two groups in terms of quantitative and ordinal measures. Odds ratios (ORs) with the corresponding 95% confidence intervals (CIs) were used to assess the strength of quantitative and binary indicators with binary outcomes.
The stepwise selection of predictors for prognostic models was performed using the Akaike information criterion (AIC). The selected predictors were included in a multi-factor logistic regression model without interactions. The Nagelkerke pseudo-R², Somers’ DXY coefficient, and C-index (area under the curve, AUC) were estimated as model quality metrics, including metrics adjusted for potential overfitting using a nonparametric bootstrap (B = 1000). The multicollinearity of predictors was assessed using the variance inflation factor (VIF). The optimal threshold value of predicted probability was determined employing Youden’s index; the prognostic accuracy, sensitivity, specificity, and positive and negative predictive values were assessed with the corresponding 95% CI.
RESULTS
Sampling
Continuous sampling was conducted according to the inclusion/exclusion criteria. Upon inclusion in the study, all participants underwent a comprehensive geriatric assessment. CRNMB detection during DOAC therapy was based on a prospective study of outpatient and inpatient medical records. The observation period lasted one year. The patients were divided into groups according to the presence or absence of CRNMB. No group had any patients who refused to participate in the study.
Characteristics of the study sample (groups)
Nonvalvular AF patients (n = 367) were selected according to the inclusion and exclusion criteria (median age of 84 [ 82; 88] years). In order to prevent thromboembolism, all participants were receiving DOACs: rivaroxaban (n = 238/367, relative proportion of 64.9%), apixaban (n = 95/367, relative proportion of 25.9%), or dabigatran etexilate (n = 34/367, relative proportion of 9.2%). The main group included 195 patients with CRNMB (median age of 84 [ 82; 87] years), with women accounting for 66.7%. The control group consisted of 172 patients without CRNMB (median age of 84 [ 82; 88] years), with women accounting for 72.7%. No significant differences were found between the groups in terms of sex, age, risk of ischemic stroke on the CHA2DS2-VASc (Congestive heart failure; Hypertension; Age; Diabetes mellitus; Prior Stroke or TIA or Thromboembolism; Vascular disease; Age 65–74 years; Sex category) scale, risk of bleeding on the HAS-BLED scale, body mass index, and key bleeding risk factors according to the criteria of clinical guidelines for the treatment of atrial fibrillation and flutter of the Russian Society of Cardiology [1][20].
Main study results
Analysis of geriatric factors affecting the development of clinically relevant non-major bleeding
The social domain was assessed by studying 24 parameters, including the presence of a disability; the presence of a partner or spouse; the presence of children; marital status; social circle and social contacts; living conditions; financial capabilities; work activity; profession; education; religiosity; the need for outpatient, inpatient, routine, or emergency medical care; preferential provision of medicines. The patients with CRNMB exhibited higher social engagement as compared to the patients without CRNMB. For example, it was statistically significantly more common for the patients with CRNMB to live in a family (p = 0.031); living alone was statistically significantly associated with 1.65-time lower odds (95% CI: 1.08; 2.51) of having CRNMB as compared to living in a family (p = 0.025). As compared to the patients without CRNMB, the CRNMB patients had a statistically significantly higher number of household members (p = 0.003); on average, a one-person increase in the number of household members was statistically significantly associated with 1.22-time higher odds (95% CI: 1.03; 1.47) of having CRNMB (p = 0.025). It was statistically significantly more common for the patients with CRNMB to be married (p = 0.014) and attend church (p = 0.046); regular church attendance (mosque, synagogue, Buddhist temple, or place of worship) was found to be associated with 3.75-time higher odds (95% CI: 1.23; 11.5) of having CRNMB (p = 0.025) (Table 1).
Table 1. Geriatric risk factors: social domain
Таблица 1. Гериатрические факторы риска: социальный домен
|
Parameter |
All patients (n = 367) |
Clinically relevant non-major bleeding |
Level of statistical significance, p |
|
|
non-present (n = 172) |
present (n = 195) |
|||
|
Disability, n (%) |
304/359 (84.7%) |
141/169 (83.4%) |
163/190 (85.8%) |
0.536 |
|
Class 1, n (%) |
19/359 (5.3%) |
9/169 (5.3%) |
10/190 (5.3%) |
0.999 |
|
Class 2, n (%) |
223/359 (62.1%) |
100/169 (59.2%) |
123/190 (64.7%) |
0.39 |
|
Class 3, n (%) |
62/359 (17.3%) |
32/169 (18.9%) |
30/190 (15.8%) |
0.495 |
|
Type of living arrangement |
||||
|
independent living, n (%) |
155/363 (42.7%) |
84/171 (49.1%) |
71/192 (37%) |
0.034 |
|
with family, n (%) |
206/363 (56.7%) |
86/171 (50.3%) |
120/192 (62.5%) |
0.021 |
|
nursing home, n (%) |
2/363 (0.6%) |
1/171 (0.6%) |
1/192 (0.5%) |
0.728 |
|
Number of household members, persons, Me (Q1; Q3) |
1 (1; 2) |
1 (1; 2) |
2 (1; 2) |
0.003 |
|
Marital status |
||||
|
married, n (%) |
81/362 (22.4%) |
31/171 (18.1%) |
50/191 (26.2%) |
0.103 |
|
widowed, n (%) |
257/362 (71%) |
130/171 (76%) |
127/191 (66.5%) |
0.039 |
|
divorced/separated, n (%) |
18/362 (5%) |
5/171 (2.9%) |
13/191 (6.8%) |
0.155 |
|
single, n (%) |
4/362 (1.1%) |
4/171 (2.3%) |
0/191 (0%) |
0.047 |
|
common law marriage, n (%) |
2/362 (0.6%) |
1/171 (0.6%) |
1/191 (0.5%) |
0.999 |
|
Presence of children, n (%) |
334/364 (91.8%) |
156/171 (91.2%) |
178/193 (92.2%) |
0.729 |
|
Number of children, persons, Me (Q1; Q3) |
1 (1; 2) |
1 (1; 2) |
1 (1; 2) |
0.645 |
|
Education |
||||
|
no formal education, n (%) |
2/362 (0.6%) |
0/170 (0%) |
2/192 (1%) |
0.501 |
|
primary / incomplete primary education, n (%) |
45/362 (12.4%) |
24/170 (14.1%) |
21/192 (10.9%) |
0.442 |
|
secondary/incomplete secondary education, n (%) |
70/362 (19.3%) |
28/170 (16.5%) |
42/192 (21.9%) |
0.252 |
|
technical education, n (%) |
98/362 (27.1%) |
48/170 (28.2%) |
50/192 (26%) |
0.710 |
|
higher education / incomplete degree, n (%) |
129/362 (35.6%) |
61/170 (35.9%) |
68/192 (35.4%) |
0.993 |
|
higher education + academic degree, n (%) |
18/362 (5%) |
9/170 (5.3%) |
9/192 (4.7%) |
0.975 |
|
Medical house calls, cases, Me (Q1; Q3) |
0 (0; 2) |
1 (0; 2.8) |
0 (0; 2) |
0.118 |
|
Visits to the outpatient clinic, cases, Me (Q1; Q3) |
4 (1; 10) |
4 (1.0; 9.8) |
4 (1.0; 10.5) |
0.344 |
|
Ambulance calls, cases, Me (Q1; Q3) |
1 (0; 3) |
1 (0; 2) |
1 (0; 3) |
0.557 |
|
Total number of requests for medical attention, cases, Me (Q1; Q3) |
8 (4; 14) |
8 (4; 14) |
8 (4; 14) |
0.959 |
|
Number of hospitalizations, cases, Me (Q1; Q3) |
1 (1; 2) |
1 (1; 2) |
1 (1; 2) |
0.812 |
|
Reason for emergency hospitalization, n (%) |
27/342 (7.9%) |
10/161 (6.2%) |
17/181 (9.4%) |
0.276 |
|
Preferential provision of medicines, n (%) |
241/348 (69.3%) |
109/162 (67.3%) |
132/186 (71%) |
0.458 |
|
Still working, n (%) |
8/361 (2.2%) |
3/169 (1.8%) |
5/192 (2.6%) |
0.728 |
|
Age of retirement, years, Me (Q1; Q3) |
60 (55; 69) |
60 (55; 70) |
60 (55; 68) |
0.887 |
|
Financial capabilities |
||||
|
low, n (%) |
36/363 (9.9%) |
18/170 (10.6%) |
18/193 (9.3%) |
0.825 |
|
medium, n (%) |
324/363 (89.3%) |
150/170 (88.2%) |
174/193 (90.2%) |
0.661 |
|
high, n (%) |
3/363 (0.8%) |
2/170 (1.2%) |
1/193 (0.5%) |
0.602 |
|
Religion/faith |
||||
|
atheist, n (%) |
39/362 (10.8%) |
19/170 (11.2%) |
20/192 (10.4%) |
0.940 |
|
Eastern Orthodoxy, n (%) |
268/362 (74%) |
121/170 (71.2%) |
147/192 (76.6%) |
0.334 |
|
Islam, n (%) |
10/362 (2.8%) |
4/170 (2.4%) |
6/192 (3.1%) |
0.755 |
|
Christianity (except for Orthodoxy), n (%) |
41/362 (11.3%) |
23/170 (13.5%) |
18/192 (9.4%) |
0.275 |
|
other, n (%) |
2/362 (0.6%) |
2/170 (1.2%) |
0/192 (0%) |
0.219 |
|
refusal to respond, n (%) |
2/362 (0.6%) |
1/170 (0.6%) |
1/192 (0.5%) |
0.999 |
|
Frequency of church attendance |
||||
|
never, n (%) |
167/363 (46%) |
80/170 (47.1%) |
87/193 (45.1%) |
0.796 |
|
sometimes, n (%) |
176/363 (48.5%) |
86/170 (50.6%) |
90/193 (46.6%) |
0.528 |
|
regularly, n (%) |
20/363 (5.5%) |
4/170 (2.4%) |
16/193 (8.3%) |
0.025 |
|
Frequency of guest visits to the patient’s home |
||||
|
not once, n (%) |
97/364 (26.6%) |
39/171 (22.8%) |
58/193 (30.1%) |
0.157 |
|
once or twice a year, n (%) |
115/364 (31.6%) |
57/171 (33.3%) |
58/193 (30.1%) |
0.557 |
|
once or twice a month, n (%) |
102/364 (28%) |
50/171 (29.2%) |
52/193 (26.9%) |
0.692 |
|
once or twice a week, n (%) |
46/364 (12.6%) |
24/171 (14%) |
22/193 (11.4%) |
0.54 |
|
every day, n (%) |
4/364 (1.1%) |
1/171 (0.6%) |
3/193 (1.6%) |
0.626 |
|
Frequency of the patient’s visits to a friend or family member |
||||
|
not once, n (%) |
141/364 (38.7%) |
66/171 (38.6%) |
75/193 (38.9%) |
0.999 |
|
once or twice a year, n (%) |
121/364 (33.2%) |
60/171 (35.1%) |
61/193 (31.6%) |
0.534 |
|
once or twice a month, n (%) |
66/364 (18.1%) |
31/171 (18.1%) |
35/193 (18.1%) |
0.999 |
|
once or twice a week, n (%) |
33/364 (9.1%) |
13/171 (7.6%) |
20/193 (10.4%) |
0.472 |
|
every day, n (%) |
3/364 (0.8%) |
1/171 (0.6%) |
2/193 (1%) |
0.999 |
|
Frequency of outings |
||||
|
not once, n (%) |
43/364 (11.8%) |
20/171 (11.7%) |
23/193 (11.9%) |
0.999 |
|
once or twice a year, n (%) |
21/364 (5.8%) |
11/171 (6.4%) |
10/193 (5.2%) |
0.767 |
|
once or twice a month, n (%) |
29/364 (8%) |
16/171 (9.4%) |
13/193 (6.7%) |
0.459 |
|
once or twice a week, n (%) |
117/364 (32.1%) |
56/171 (32.7%) |
61/193 (31.6%) |
0.881 |
|
every day, n (%) |
154/364 (42.3%) |
68/171 (39.8%) |
86/193 (44.6%) |
0.436 |
|
Internet use |
||||
|
not once, n (%) |
299/364 (82.1%) |
139/171 (81.3%) |
160/193 (82.9%) |
0.865 |
|
once or twice a year, n (%) |
9/364 (2.5%) |
5/171 (2.9%) |
4/193 (2.1%) |
0.739 |
|
once or twice a month, n (%) |
10/364 (2.7%) |
5/171 (2.9%) |
5/193 (2.6%) |
0.999 |
|
once or twice a week, n (%) |
17/364 (4.7%) |
8/171 (4.7%) |
9/193 (4.7%) |
0.999 |
|
every day, n (%) |
29/364 (8%) |
14/171 (8.2%) |
15/193 (7.8%) |
0.999 |
|
Telephone and/or Internet communication |
||||
|
not once, n (%) |
23/364 (6.3%) |
9/171 (5.3%) |
14/193 (7.3%) |
0.581 |
|
once or twice a year, n (%) |
9/364 (2.5%) |
2/171 (1.2%) |
7/193 (3.6%) |
0.182 |
|
once or twice a month, n (%) |
15/364 (4.1%) |
10/171 (5.8%) |
5/193 (2.6%) |
0.192 |
|
once or twice a week, n (%) |
60/364 (16.5%) |
36/171 (21.1%) |
24/193 (12.4%) |
0.037 |
|
every day, n (%) |
257/364 (70.6%) |
114/171 (66.7%) |
143/193 (74.1%) |
0.175 |
Notes: The table was compiled by the authors; p — the values were obtained by comparing the two groups using the Mann—Whitney test (for quantitative indicators), as well as Fisher’s exact test and Pearson’s χ² test (for categorical indicators); the table indicates the number of patients included in the analysis.
Примечания: таблица составлена авторами; p — значения получены при сравнении двух групп с использованием теста Манна — Уитни (для количественных показателей), а также точного теста Фишера и теста χ² Пирсона (для категориальных показателей); в таблице указано количество пациентов, которые были включены в анализ.
The mental and emotional domain was analyzed using ten scales that cover cognitive functions and emotional state. As compared to the patients without CRNMB, the CRNMB patients were found to be more emotionally stable. For example, the patients with CRNMB had statistically significantly lower scores on the GDS-15 and GDS-30 scales (p = 0.007 and 0.027, respectively); on average, every 1-point increase in the GDS-15 scale score was associated with 1.08-time lower odds (95% CI: 1.02; 1.14) of having CRNMB (p = 0.01), and every 1-point increase in the GDS-30 scale score was associated with 1.04-time lower odds (95% CI: 1.00; 1.07) of having CRNMB (p = 0.032) (Table 2).
Table 2. Geriatric risk factors: mental and emotional status
Таблица 2. Гериатрические факторы риска: психоэмоциональный статус
|
Parameter |
All patients (n = 367) |
Clinically relevant non-major bleeding |
Level of statistical significance, p |
|
|
non-present (n = 172) |
present (n = 195) |
|||
|
Mini-Cog screening test: word recall, points, Me (Q1; Q3) |
2 (1; 3) |
2 (1; 3) |
2 (1; 3) |
0.910 |
|
Mini-Cog screening test: clock drawing test, points, Me (Q1; Q3) |
1 (1; 2) |
1 (1; 2) |
1 (1; 2) |
0.269 |
|
Mini-Cog screening test: total, points, Me (Q1; Q3) |
3 (2; 4) |
3 (2; 4) |
3 (2; 4) |
0.597 |
|
Mini-Cog screening test |
||||
|
• presence of cognitive impairment (score of 0–3), n (%) |
217/367 (59.1%) |
103/172 (59.9%) |
114/195 (58.5%) |
0.782 |
|
• no cognitive impairment (score of 4–5), n (%) |
150/367 (40.9%) |
69/172 (40.1%) |
81/195 (41.5%) |
0.782 |
|
Mini-mental State Examination, points, Me (Q1; Q3) |
26 (23; 27.5) |
26 (23; 28) |
26 (23; 27) |
0.597 |
|
Mini-mental State Examination |
||||
|
• no cognitive impairment (score of 28–30), n (%) |
88/351 (25.1%) |
44/161 (27.3%) |
44/190 (23.2%) |
0.674 |
|
• pre-dementia cognitive impairment (score of 24–27), n (%) |
136/351 (38.7%) |
59/161 (36.6%) |
77/190 (40.5%) |
0.674 |
|
• mild dementia (score of 20–23), n (%) |
96/351 (27.4%) |
43/161 (26.7%) |
53/190 (27.9%) |
0.674 |
|
• moderate dementia (score of 11–19), n (%) |
30/351 (8.5%) |
14/161 (8.7%) |
16/190 (8.4%) |
0.674 |
|
• severe dementia (score of 0–10), n (%) |
1/351 (0.3%) |
1/161 (0.6%) |
0/190 (0%) |
0.674 |
|
Montreal Cognitive Assessment, points, Me (Q1; Q3) |
22 (19; 25) |
23 (18.8; 25.0) |
22 (20; 25) |
0.599 |
|
Montreal Cognitive Assessment |
||||
|
• no cognitive impairment (score of 26–30), n (%) |
75/354 (21.2%) |
36/164 (22%) |
39/190 (20.5%) |
0.744 |
|
• presence of cognitive impairment (score of 0–25), n (%) |
279/354 (78.8%) |
128/164 (78%) |
151/190 (79.5%) |
0.744 |
|
verbal fluency test: literal (VFT-L), points, Me (Q1; Q3) |
9 (6; 11) |
9 (6; 11) |
8.5 (6; 11) |
0.805 |
|
13 or more points, n (%) |
52/352 (14.8%) |
23/162 (14.2%) |
29/190 (15.3%) |
0.779 |
|
verbal fluency test: category fluency (VFT-C), points, Me (Q1; Q3) |
12 (9; 15) |
12 (8; 15) |
12 (9; 15) |
0.775 |
|
13 or more points, n (%) |
151/352 (42.9%) |
71/162 (43.8%) |
80/190 (42.1%) |
0.745 |
|
VFT-L/ VFT-C, points, Me (Q1; Q3) |
0.8 (0.5; 1) |
0.8 (0.6; 1) |
0.8 (0.5; 0.9) |
0.69 |
|
Boston Naming Test, points, Me (Q1; Q3) |
34 (28; 37) |
34 (29; 37) |
33 (28; 37) |
0.32 |
|
Literary cues, points, Me (Q1; Q3) |
4 (1; 7) |
4 (1; 7) |
4 (1; 8) |
0.313 |
|
Category cues, points, Me (Q1; Q3) |
0 (0; 4) |
0 (0; 3) |
1 (0; 5) |
0.114 |
|
Forward digit span test, points, Me (Q1; Q3) |
8 (6; 10) |
8 (6; 10) |
8 (6; 10) |
0.801 |
|
Maximum length of a sequence, points, Me (Q1; Q3) |
5 (4; 6) |
5 (4; 6) |
5 (4; 6) |
0.832 |
|
Digit Symbol Substitution Test, points, Me (Q1; Q3) |
17 (10; 21) |
17 (10; 21) |
17 (11; 21) |
0.702 |
|
10-word-list recall test: immediate recall, points, Me (Q1; Q3) |
16 (12; 19) |
16 (12; 19) |
16 (13; 19) |
0.786 |
|
10-word-list recall test: delayed recall, points, Me (Q1; Q3) |
4 (2; 5) |
4 (2; 5.3) |
4 (2; 5) |
0.541 |
|
10-word-list recall test: learning capacity, points, Me (Q1; Q3) |
8 (6; 10) |
9 (6; 10) |
8 (6; 10) |
0.473 |
|
Alzheimer’s Disease Assessment Scale-Cognitive, points, Me (Q1; Q3) |
17.4 (11.6; 25.0) |
18.2 (11.8; 25.6) |
16.4 (11.3; 24.4) |
0.505 |
|
Geriatric Depression Scale, GDS-15, points, Me (Q1; Q3) |
5 (2; 8) |
5 (3; 8) |
4 (2; 7) |
0.007 |
|
Geriatric Depression Scale, GDS-15 |
||||
|
normal (score of 0–4), n (%) |
182/367 (49.6%) |
70/172 (40.7%) |
112/195 (57.4%) |
0.001 |
|
• probable depression (score of 5–15), n (%) |
185/367 (50.4%) |
102/172 (59.3%) |
83/195 (42.6%) |
0.001 |
|
Geriatric Depression Scale, GDS-30, points, Me (Q1; Q3) |
11 (6; 16) |
12 (7.0; 16.3) |
10 (5.5; 15.0) |
0.027 |
|
Geriatric Depression Scale, GDS-30 |
||||
|
• normal (score of 0–9), n (%) |
153/351 (43.6%) |
63/164 (38.4%) |
90/187 (48.1%) |
0.171 |
|
• mild depression (score of 10–19), n (%) |
163/351 (46.4%) |
82/164 (50%) |
81/187 (43.3%) |
0.171 |
|
• severe depression (score of 20–30), n (%) |
35/351 (10%) |
19/164 (11.6%) |
16/187 (8.6%) |
0.171 |
Notes: The table was compiled by the authors; p — the values were obtained by comparing the two groups using the Mann—Whitney test (for quantitative indicators), as well as the exact test; the table indicates the number of patients included in the analysis. Abbreviations: MMSE — Mini-mental State Examination; VFT-L — verbal fluency test (literal); VFT-C — verbal fluency test (category); WLT — 10-word-list recall test; GDS — Geriatric Depression Scale.
Примечания: таблица составлена авторами; p — значения получены при сравнении двух групп с использованием теста Манна — Уитни (для количественных показателей), а также точного теста; в таблице указано количество пациентов, которые были включены в анализ. Сокращения: MMSE — краткая шкала оценки психического статуса; VFT-L — тест вербальных ассоциаций: литеральлные; VFT-C — тест вербальных ассоциаций: категориальные; WLТ — тест запоминания 10 слов; GDS — гериатрическая шкала депрессии.
Thirteen parameters were analyzed to assess functional activity and physical health, which included screening for frailty; self-assessment of quality of life and health status; basic and instrumental activities; mobility; muscle strength; nutritional status; presence of the chronic pain syndrome, orthostatic hypotension, sensory deficits, and urinary and fecal incontinence; occurrence of falls; use of assistive devices. The results revealed that compared to the patients without CRNMB, the CRNMB patients were less likely to have frailty and use assistive devices, rated their quality of life and health status higher, were more active in performing activities of daily living, and were more mobile. For example, the patients with CRNMB exhibited better mood (OR = 0.65 (95% CI: 0.43; 0.99), p = 0.043), and it was statistically significantly more uncommon for them to have frailty according to the Age is No Barrier questionnaire (p = 0.015). As compared to the patients without CRNMB, the CRNMB patients had higher VAS scores measuring quality of life and health status (p = 0.053 and 0.05, respectively); on average, every 1-point increase in the VAS quality of life score was associated with 1.09-time higher odds (95% CI: 0.99; 1.21) of having a bleeding event (p = 0.084); every 1-point increase in the health status score, 1.11-time higher odds (95% CI: 0.99; 1.26) (p = 0.07). The patients with CRNMB had statistically significantly higher Lawton IADL scores (p = 0.004); on average, every 1-point increase in the score was associated with 1.17-time higher odds (95% CI: 1.04; 1.32) of having CRNMB (p = 0.008). The presence of CRNMB was associated with a longer tandem stance time (p = 0.014); on average, for every one-second increase, the odds of having CRNMB increased by 1.05 times (95% CI: 1.01; 1.10) (p = 0.013). The CRNMB patients had statistically significantly lower scores on the 5-time chair stand test and Timed Up and Go Test as compared to patients without CRNMB (p = 0.045 and 0.025, respectively); on average, every 10-point increase in scores on these scales was associated with 1.16-time (95% CI: 1.01; 1.34) (p = 0.045) and 1.23-time lower odds (95% CI: 1.02; 1.50) of having CRNMB (p = 0.03), respectively (Table 3).
Table 3. Geriatric risk factors: functional and physical status
Таблица 3. Гериатрические факторы риска: функциональный и физический статус
|
Parameter |
All patients (n = 367) |
Clinically relevant non-major bleeding |
Level of statistical significance, p |
|
|
non-present (n = 172) |
present (n = 195) |
|||
|
Age is No Barrier questionnaire (weight), n (%) |
117/364 (32.1%) |
60/171 (35.1%) |
57/193 (29.5%) |
0.257 |
|
Age is No Barrier questionnaire (deterioration of vision or hearing), n (%) |
298/364 (81.9%) |
144/171 (84.2%) |
154/193 (79.8%) |
0.275 |
|
Age is No Barrier questionnaire (fall-related injuries), n (%) |
106/363 (29.2%) |
47/170 (27.6%) |
59/193 (30.6%) |
0.541 |
|
Age is No Barrier questionnaire (mood), n (%) |
186/364 (51.1%) |
97/171 (56.7%) |
89/193 (46.1%) |
0.043 |
|
Age is No Barrier questionnaire (memory problem), n (%) |
240/363 (66.1%) |
111/170 (65.3%) |
129/193 (66.8%) |
0.756 |
|
Age is No Barrier questionnaire (urinary incontinence), n (%) |
206/364 (56.6%) |
97/171 (56.7%) |
109/193 (56.5%) |
0.962 |
|
Age is No Barrier questionnaire (walking difficulty), n (%) |
218/364 (59.9%) |
106/171 (62%) |
112/193 (58%) |
0.442 |
|
Age is No Barrier questionnaire, points, Me (Q1; Q3) |
4 (3; 5) |
4 (3; 5) |
4 (2; 5) |
0.245 |
|
Age is No Barrier questionnaire |
||||
|
• fit (score of 0–2), n (%) |
86/364 (23.6%) |
29/171 (17%) |
57/193 (29.5%) |
0.008 |
|
• less fit (score of 3–4), n (%) |
151/364 (41.5%) |
80/171 (46.8%) |
71/193 (36.8%) |
0.063 |
|
• frail (score of 5–7), n (%) |
127/364 (34.9%) |
62/171 (36.3%) |
65/193 (33.7%) |
0.663 |
|
Self-assessment of quality of life on the visual analogue scale, points, Me (Q1; Q3) |
6 (5; 8) |
6 (4; 7.5) |
6 (5; 8) |
0.053 |
|
Self-assessment of health status on the visual analogue scale, points, Me (Q1; Q3) |
5 (4; 6) |
5 (4; 6) |
5 (4; 7) |
0.050 |
|
Barthel Index for Activities of Daily Living, points, Me (Q1; Q3) |
90 (85; 95) |
90 (80; 95) |
90 (85; 95) |
0.380 |
|
Barthel Index for Activities of Daily Living |
||||
|
• total independence (score of 96–100), n (%) |
67/364 (18.4%) |
34/171 (19.9%) |
33/193 (17.1%) |
0.570 |
|
• slight dependence (score of 91–95), n (%) |
80/364 (22%) |
35/171 (20.5%) |
45/193 (23.3%) |
0.614 |
|
• moderate dependence (score of 61–90), n (%) |
203/364 (55.8%) |
94/171 (55%) |
109/193 (56.5%) |
0.893 |
|
• severe dependence (score of 21–60), n (%) |
14/364 (3.8%) |
8/171 (4.7%) |
6/193 (3.1%) |
0.608 |
|
• total dependence (score of 0–20), n (%) |
0/364 (0%) |
0/171 (0%) |
0/193 (0%) |
– |
|
Lawton IADL scale, points, Me (Q1; Q3) |
7 (5; 8) |
6 (5; 7.5) |
7 (5; 8) |
0.004 |
|
Functional Activities Questionnaire, points, Me (Q1; Q3) |
8 (4; 14) |
9 (5; 15) |
8 (3; 13) |
0.106 |
|
Mini Nutritional Assessment, points, Me (Q1; Q3) |
22 (19.5; 24) |
22 (19.5; 24) |
22.5 (20; 24.5) |
0.117 |
|
Mini Nutritional Assessment |
||||
|
• normal nutritional status (23.5–30 points), n (%) |
121/350 (34.6%) |
51/164 (31.1%) |
70/186 (37.6%) |
0.246 |
|
• at risk of malnutrition (17–23.5 points), n (%) |
204/350 (58.3%) |
102/164 (62.2%) |
102/186 (54.8%) |
0.215 |
|
• malnourished (less than 17 points), n (%) |
25/350 (7.1%) |
11/164 (6.7%) |
14/186 (7.5%) |
0.928 |
|
Short Physical Performance Battery, points, Me (Q1; Q3) |
5 (3; 6) |
4 (3; 6) |
5 (3; 6) |
0.999 |
|
Short Physical Performance Battery |
||||
|
• fit (score of 10–12), n (%) |
18/360 (5%) |
5/169 (3%) |
13/191 (6.8%) |
0.155 |
|
• less fit (score of 8–9), n (%) |
41/360 (11.4%) |
19/169 (11.2%) |
22/191 (11.5%) |
>0.999 |
|
• frail (7 or less points), n (%) |
301/360 (83.6%) |
145/169 (85.8%) |
156/191 (81.7%) |
0.35 |
|
Tandem stance, seconds, Me (Q1; Q3) |
2 (0; 6.5) |
1 (0; 4.2) |
2.3 (0; 8.5) |
0.014 |
|
four-meter walk test, seconds, Me (Q1; Q3) |
9 (6.7; 15.3) |
9.5 (7.0; 15.3) |
8.9 (6.3; 14.9) |
0.27 |
|
walking speed, m/s, Me (Q1; Q3) |
0.4 (0.3; 0.6) |
0.4 (0.3; 0.6) |
0.4 (0.3; 0.6) |
0.552 |
|
five-time chair stand test, seconds, Me (Q1; Q3) |
15.9 (0; 24.5) |
17.7 (3.5; 26.3) |
15.5 (0; 20.5) |
0.045 |
|
Five-time chair stand test |
||||
|
• unable to perform the test or over 60 s, n (%) |
100/359 (27.9%) |
47/169 (27.8%) |
53/190 (27.9%) |
0.999 |
|
• over 16.7 seconds, n (%) |
168/359 (46.8%) |
81/169 (47.9%) |
87/190 (45.8%) |
0.711 |
|
• 13.7–16.69 s, n (%) |
48/359 (13.4%) |
24/169 (14.2%) |
24/190 (12.6%) |
0.755 |
|
• 11.2–13.69 s n (%) |
27/359 (7.5%) |
13/169 (7.7%) |
14/190 (7.4%) |
0.999 |
|
• 11.19 s or less, n (%) |
16/359 (4.5%) |
4/169 (2.4%) |
12/190 (6.3%) |
0.125 |
|
Timed Up and Go Test, seconds, Me (Q1; Q3) |
16.1 (12.0; 21.4) |
17.1 (12.9; 22.8) |
15.3 (11.5; 20.4) |
0.025 |
|
Timed Up and Go Test |
||||
|
• normal (10 s or less), n (%) |
59/348 (17%) |
26/163 (16%) |
33/185 (17.8%) |
0.743 |
|
• intermediate (11–13 s), n (%) |
289/348 (83%) |
137/163 (84%) |
152/185 (82.2%) |
0.787 |
|
• gait and balance abnormalities (14 s и and more), n (%) |
0/348 (0%) |
0/163 (0%) |
0/185 (0%) |
– |
|
single-leg stance test, seconds, Me (Q1; Q3) |
1.2 (0; 2.8) |
1.0 (0; 2.6) |
1.5 (0; 3) |
0.321 |
|
Dynamometry: handgrip strength, kg, Me (Q1; Q3) |
12.5 (9.0; 17.5) |
12.5 (9.2; 17.3) |
12.3 (9.0; 17.9) |
0.869 |
|
handgrip strength (women), kg, Me (Q1; Q3) |
10.5 (7.8; 13.3) |
10.5 (8.0; 14.0) |
10.3 (7.5; 13.0) |
0.410 |
|
handgrip strength (men), kg, Me (Q1; Q3) |
19.8 (16.4; 22.6) |
19.8 (17.6; 2.02) |
19.3 (16.3; 23.3) |
0.953 |
|
decrease in handgrip strength, n (%) |
352/362 (97.2%) |
166/170 (97.6%) |
186/192 (96.9%) |
0.755 |
|
decrease in handgrip strength (women), n (%) |
243/250 (97.2%) |
120/123 (97.6%) |
123/127 (96.9%) |
0.999 |
|
decrease in handgrip strength (men), n (%) |
109/112 (97.3%) |
46/47 (97.9%) |
63/65 (96.9%) |
0.999 |
|
orthostatic hypotension, n (%) |
72/363 (19.8%) |
34/171 (19.9%) |
38/192 (19.8%) |
0.983 |
|
Precursors of orthostatic hypotension, n (%) |
199/356 (55.9%) |
95/168 (56.5%) |
104/188 (55.3%) |
0.816 |
|
hearing impairment, n (%) |
294/364 (80.8%) |
136/171 (79.5%) |
158/193 (81.9%) |
0.573 |
|
visual deficit, n (%) |
357/361 (98.9%) |
169/170 (99.4%) |
188/191 (98.4%) |
0.625 |
|
chronic pain, n (%) |
340/363 (93.7%) |
161/170 (94.7%) |
179/193 (92.7%) |
0.444 |
|
urinary incontinence, n (%) |
212/364 (58.2%) |
97/171 (56.7%) |
115/193 (59.6%) |
0.581 |
|
difficult urination, n (%) |
86/349 (24.6%) |
42/163 (25.8%) |
44/186 (23.7%) |
0.648 |
|
nocturia, n (%) |
316/351 (90%) |
150/164 (91.5%) |
166/187 (88.8%) |
0.401 |
|
fecal incontinence, n (%) |
37/362 (10.2%) |
16/170 (9.4%) |
21/192 (10.9%) |
0.632 |
|
falls in the past year, n (%) |
158/363 (43.5%) |
70/171 (40.9%) |
88/192 (45.8%) |
0.347 |
|
Number of falls in the past year |
||||
|
• no falls, n (%) |
205/363 (56.5%) |
101/171 (59.1%) |
104/192 (54.2%) |
0.351 |
|
• one fall, n (%) |
66/363 (18.2%) |
29/171 (17%) |
37/192 (19.3%) |
0.697 |
|
• two or more, n (%) |
92/363 (25.3%) |
41/171 (24%) |
51/192 (26.6%) |
0.696 |
Notes: The table was compiled by the authors; p — the values were obtained by comparing the two groups using the Mann—Whitney test (for quantitative indicators), as well as Fisher’s exact test and Pearson’s χ² test (for categorical indicators); the table indicates the number of patients included in the analysis.
Примечания: таблица составлена авторами; p — значения получены при сравнении двух групп с использованием теста Манна — Уитни (для количественных показателей), а также точного теста Фишера и теста χ² Пирсона (для категориальных показателей); в таблице указано количество пациентов, которые были включены в анализ.
The use of assistive devices was statistically significantly less common in the patients with CRNMB as compared to the patients without CRNMB (p = 0.022) (Table 4).
Table 4. Geriatric risk factors: physical status (use of assistive devices)
Таблица 4. Гериатрические факторы риска: физический статус (использование вспомогательных средств)
|
Parameter |
All patients (n = 367) |
Clinically relevant non-major bleeding |
Level of statistical significance, p |
|
|
non-present (n = 172) |
present (n = 195) |
|||
|
Use of glasses/lenses, n (%) |
293/364 (80.5%) |
142/171 (83%) |
151/193 (78.2%) |
0.248 |
|
Use of a hearing aid, n (%) |
35/364 (9.6%) |
19/171 (11.1%) |
16/193 (8.3%) |
0.362 |
|
Use of prosthetic denture, n (%) |
268/364 (73.6%) |
120/171 (70.2%) |
148/193 (76.7%) |
0.160 |
|
Use of a cane, n (%) |
210/364 (57.7%) |
103/171 (60.2%) |
107/193 (55.4%) |
0.356 |
|
Use of crutches, n (%) |
10/364 (2.7%) |
7/171 (4.1%) |
3/193 (1.6%) |
0.200 |
|
Use of a walker, n (%) |
28/364 (7.7%) |
17/171 (9.9%) |
11/193 (5.7%) |
0.130 |
|
Use of walking aids, n (%) |
222/363 (61.2%) |
109/171 (63.7%) |
113/192 (58.9%) |
0.340 |
|
Use of orthopedic footwear, n (%) |
42/363 (11.6%) |
19/170 (11.2%) |
23/193 (11.9%) |
0.826 |
|
Use of orthopedic insoles, n (%) |
67/364 (18.4%) |
28/171 (16.4%) |
39/193 (20.2%) |
0.346 |
|
Use of an orthopedic corset, n (%) |
32/364 (8.8%) |
16/171 (9.4%) |
16/193 (8.3%) |
0.720 |
|
Use of incontinence pads, n (%) |
134/363 (36.9%) |
68/170 (40%) |
66/193 (34.2%) |
0.253 |
|
Use of diapers or underpads, n (%) |
37/364 (10.2%) |
20/171 (11.7%) |
17/193 (8.8%) |
0.363 |
|
Number of assistive devices, pcs, Me (Q1; Q3) |
3 (2; 4) |
3 (2; 4) |
3 (2; 4) |
0.316 |
|
Use of any assistive device, n (%) |
354/364 (97.3%) |
170/171 (99.4%) |
184/193 (95.3%) |
0.022 |
Notes: The table was compiled by the authors; p — the values were obtained by comparing the two groups using the Mann—Whitney test (for quantitative indicators), as well as Fisher’s exact test and Pearson’s χ² test (for categorical indicators); the table indicates the number of patients included in the analysis.
Примечания: таблица составлена авторами; p — значения получены при сравнении двух групп с использованием теста Манна — Уитни (для количественных показателей), а также точного теста Фишера и теста χ² Пирсона (для категориальных показателей); в таблице указано количество пациентов, которые были включены в анализ.
The frailty index was calculated using a deficit accumulation model, which involved the assessment of 46 deficits on the Rockwood scale [18] and 34 deficits on the scale used in the SPRINT study [19]. An analysis of frailty indices revealed that the CRNMB patients were less frail compared to the patients without CRNMB according to the frailty index used in the SPRINT study. For example, a statistically significant association was found between the presence of CRNMB and lower SPRINT scores (p = 0.005); on average, every 0.1-point increase in the score was associated with a decrease in the odds of having CRNMB by 1.46 [ 1.14; 1.88] times (p = 0.003) (Table 5).
Table 5. Geriatric risk factors: frailty indices
Таблица 5. Гериатрические факторы риска: индексы хрупкости
|
Parameter |
All patients (n = 367) |
Clinically relevant non-major bleeding |
Level of statistical significance, p |
|
|
non-present (n = 172) |
present (n = 195) |
|||
|
Rockwood frailty index |
0.36 (0.29; 0.44) |
0.37 (0.31; 0.44) |
0.35 (0.29; 0.43) |
0.172 |
|
Frailty status on the Rockwood scale |
||||
|
• fit (≤ 0.1) |
0/367 (0%) |
0/172 (0%) |
0/195 (0%) |
– |
|
• less fit (0.11–0.20) |
13/367 (3.5%) |
6/172 (3.5%) |
7/195 (3.6%) |
0.999 |
|
• frail (≥ 0.21) |
354/367 (96.5%) |
166/172 (96,5%) |
188/195 (96.4%) |
0.999 |
|
• SPRINT frailty index |
0.37 (0.31; 0.43) |
0.39 (0.33; 0.44) |
0.36 (0.31; 0.42) |
0.005 |
|
Frailty status on the SPRINT scale |
||||
|
• fit (≤ 0.1) |
0/367 (0%) |
0/172 (0%) |
0/195 (0%) |
– |
|
• less fit (0.11–0.20) |
5/367 (1.4%) |
1/172 (0.6%) |
4/195 (2.1%) |
0.377 |
|
• frail (≥ 0.21) |
362/367 (98.6%) |
171/172 (99.4%) |
191/195 (97.9%) |
0.377 |
Notes: The table was compiled by the authors; p — the values were obtained by comparing the two groups using the Mann—Whitney test (for quantitative indicators), as well as Fisher’s exact test and Pearson’s χ² test (for categorical indicators). Abbreviations: SPRINT — Systolic Blood Pressure Intervention Trial.
Примечания: таблица составлена авторами; p — значения получены при сравнении двух групп с использованием теста Манна — Уитни (для количественных показателей), а также точного теста Фишера и теста χ² Пирсона (для категориальных показателей). Cокращение: SPRINT — шкала оценки индекса хрупкости.
This study presents a systems approach to comprehensive geriatric assessment that relies on the analysis of four key domains: social functioning; mental and emotional status; physical health; functional capacity. The research design enables a comprehensive status analysis of elderly patients, taking the modern geriatric principles into account. The findings provide a holistic understanding of the relationships between different aspects of geriatric status, which is essential for the development of personalized approaches in geriatric practice.
Multi-factor analysis predicting the probability of clinically relevant non-major bleeding events
Table 6 presents a multi-factor model for predicting the probability of CRNMB, developed using a stepwise selection of predictors, with their exclusion according to the Akaike information criterion (AIC). The resulting model was characterized by a Nagelkerke pseudo-R² value of 0.1 (adjusted value of 0.05), a Somers’ DXY coefficient of 0.27 (adjusted value of 0.23), and an AUC of 0.64 [ 95% CI: 0.58; 0.69] (adjusted value of 0.61) (Figs 2, 3). The coefficients of the model were used to develop a prognostic nomogram to estimate the probability of CRNMB on DOACs (Fig. 4).
Table 6. Coefficients in the resulting model for predicting the probability of clinically relevant non-major bleeding
Таблица 6. Коэффициенты в полученной модели прогнозирования вероятности клинически значимого небольшого кровотечения
|
Predictor |
β (SE) |
OR |
95% CI |
p |
VIF |
|
Intercept term |
2.15 (1.62) |
– |
– |
– |
– |
|
Number of household members |
0.28 (0.1) |
1.32 |
1.10; 1.62 |
0.005 |
1.06 |
|
Barthel ADL score |
−0.02 (0.01) |
0.98 |
0.96; 1.01 |
0.239 |
1.65 |
|
Lawton IADL score |
0.21 (0.08) |
1.24 |
1.06; 1.45 |
0.008 |
1.53 |
|
Timed Up and Go Test (seconds) |
−0.01 (0.01) |
0.99 |
0.96; 1.01 |
0.237 |
1.35 |
|
Use of any assistive device |
−2.3 (1.08) |
0.10 |
0.01; 0.57 |
0.033 |
1.02 |
Notes: The table was compiled by the authors; p — the values were obtained using the Wald test. Abbreviations: CI — confidence interval; OR — odds ratio; SE — standard error; VIF — variance inflation factor.
Примечания: таблица составлена авторами; p — значения получены с использованием теста Вальда. Сокращения: CI — доверительный интервал; OR — отношение шансов; SE — standard error, стандартная ошибка; VIF — variance inflation factor, фактор инфляции дисперсии.
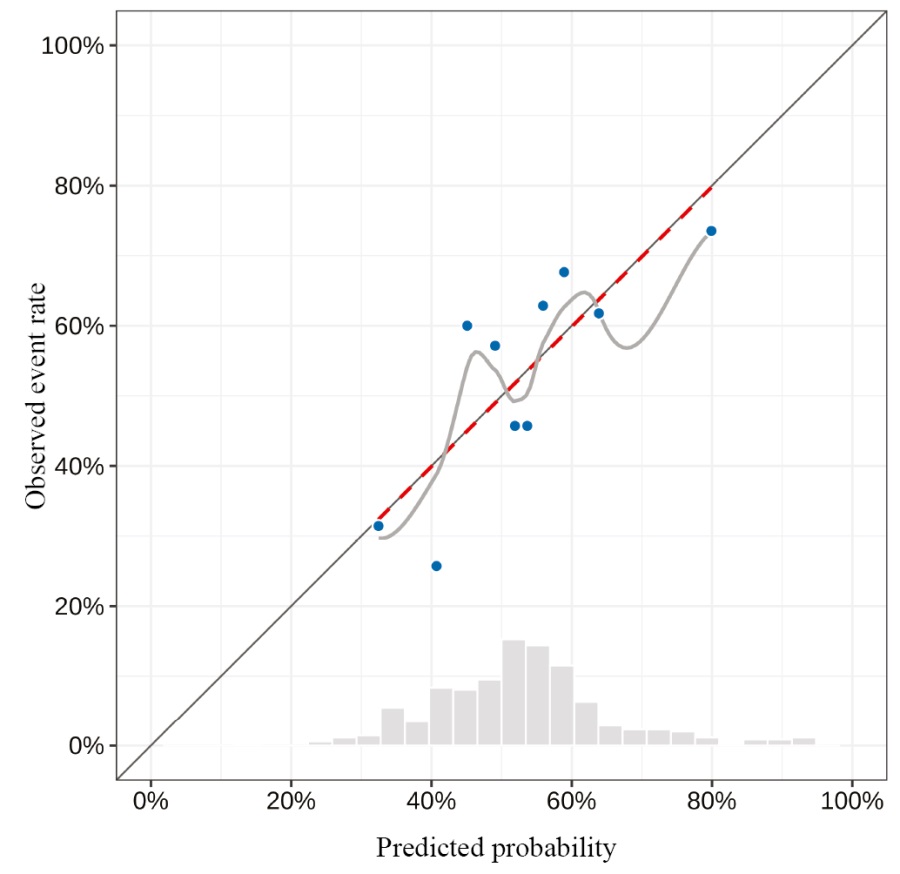
Fig. 2. Calibration curve for the predictions obtained using the model
Note: performed by the authors.
Рис 2. Калибровочная кривая для предсказаний, полученных с использованием модели
Примечание: рисунок выполнен авторами.
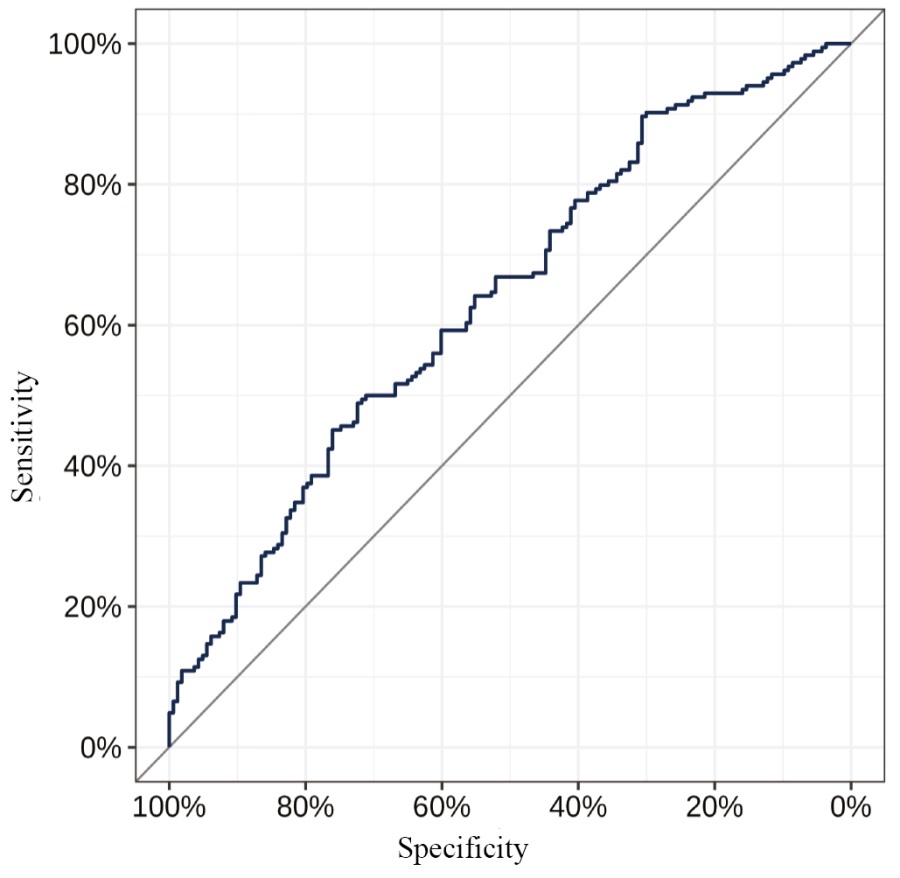
Fig. 3. ROC curve for the predictions obtained using the model
Note: performed by the authors.
Рис 3. ROC-кривая для предсказаний, полученных с использованием модели
Примечание: рисунок выполнен авторами.
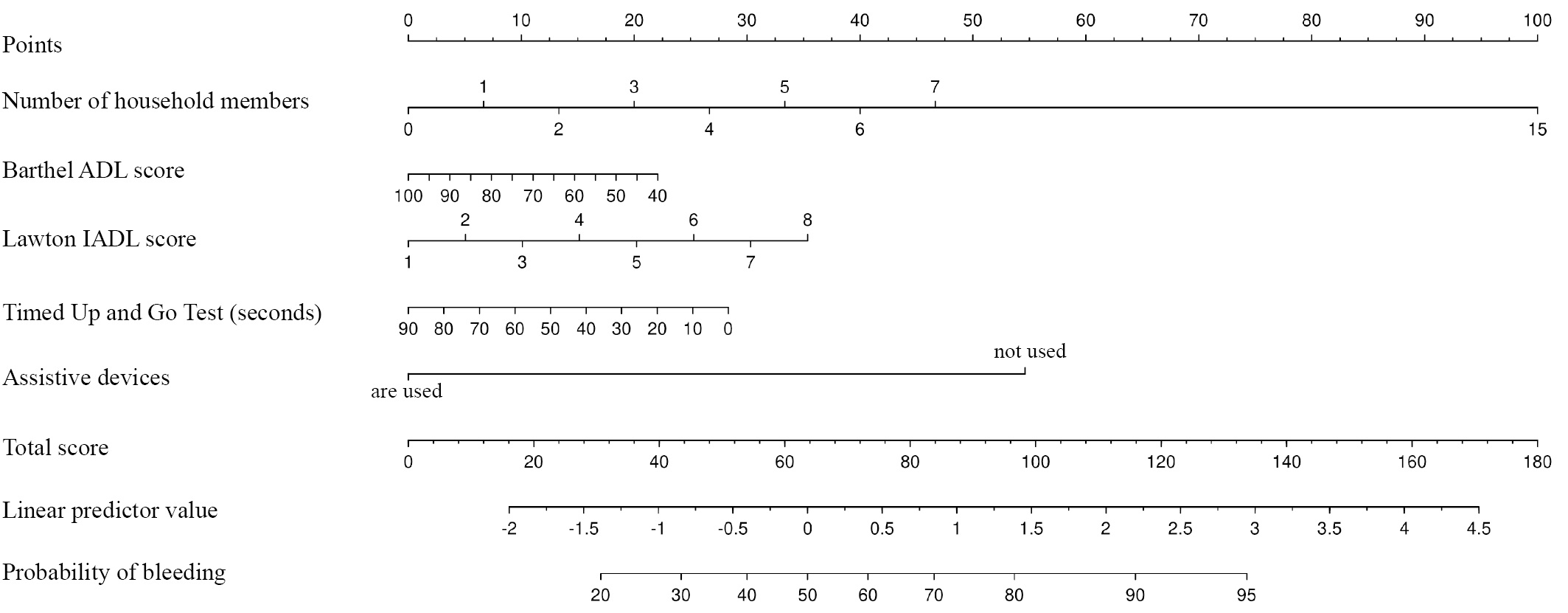
Fig. 4. Nomogram to predict the probability of clinically relevant non-major bleeding
Notes: performed by the authors; to estimate the probability of an event, it is necessary to determine the score corresponding to the value of the predictor by dropping the normal to the appropriate scale; then, it is necessary to find the total score and, by dropping the normal to the appropriate scale, estimate the linear predictor (log odds) and the probability of the event.
Рис. 4. Номограмма для прогнозирования вероятности развития клинически значимого небольшого кровотечения
Примечания: рисунок выполнен авторами; для оценки вероятности события необходимо определить балл, соответствующий значению предиктора, опустив нормаль на соответствующую шкалу, затем необходимо найти сумму баллов и, опустив нормаль на соответствующую шкалу, найти оценку значения линейного предиктора (логарифма шансов события) и вероятности события.
At a threshold predicted CRNMB probability of 55%, the resulting model was characterized by a prognostic accuracy of 59.9% [ 95% CI: 54.6; 65.1], a sensitivity of 48.9% [ 95% CI: 41.5; 56.4], and a specificity of 72.4% [ 95% CI: 64.9; 79.1], with a positive predictive value of 66.7% [ 95% CI: 58; 74.5] and a negative predictive value of 55.7% [ 95% CI: 48.7; 62.5].
Additional study results
No additional results were obtained during the study.
DISCUSSION
Summary of the main study result
The analysis of geriatric predictors of CRNMB on anticoagulants in AF patients aged ≥80 years identified significant factors in all four domains of comprehensive geriatric assessment: social; mental and emotional; physical; functional.
The performed multiple-factor regression analysis provided a means to develop a prognostic CRNMB risk model. The obtained coefficients were used to develop a prognostic nomogram to estimate the probability of CRNMB in patients receiving DOACs. Significant prognostic factors included the number of household members, Barthel ADL score, Lawton IADL score, Timed Up and Go Test, and use of assistive devices. The developed model was characterized by a prognostic accuracy of 59.9%, a sensitivity of 48.9%, and a specificity of 72.4%. The positive predictive value amounted to 66.7%; the negative predictive value, 55.7%.
Research limitations
The main limitations of the study include the small sample size and the associated lack of statistical power. Also, this observational study of real clinical practice does not provide a means to assess patient compliance with regard to DOAC therapy and the presence of CRNMB in frail patients (or patients with frailty); however, it reflects the actual health care practice.
Interpretation of the study results
In the social domain, the study showed that it was more common for the CRNMB patients to live in a large household and to attend church. High social engagement contributes to CRNMB detection: friends, coworkers, and relatives may notice the signs of CRNMB and pay more attention to them than the patients themselves, which makes the patient seek the help of specialists who can diagnose CRNMB.
The assessment of mental and emotional status showed that the patients with CRNMB were not prone to depression and had lower scores on the GDS-15 and GDS-30 scales. This can be attributed to the fact that depressed patients may be less likely to complain, including about CRNMB, and as a consequence, may not mention something relevant at a doctor’s appointment [3].
The assessment of functional and physical status revealed that the patients with CRNMB were more active in performing their daily living activities (high Lawton IADL score), more mobile (according to balance tests, i.e., tandem stance, 5-time chair stand test, and Timed Up and Go Test), and it was less common for them to use assistive devices, which could lead to collisions and falls, resulting in CRNMB. In addition, it was less common for the CRNMB patients to have frailty according to the Age is No Barrier questionnaire, and they were less frail according to the frailty index used in the SPRINT study [19][20].
Thus, the present study showed that the group of CRNMB patients included fewer frail patients and those in need of assistance since in actual clinical practice, patients with frailty are less likely to seek the help of specialists despite the presence of CRNMB. On the other hand, self-reliant patients are under less supervision, which may lead to injuries and the development of CRNMB.
The findings differ from the results of other studies [7–11]. For example, Brook et. al [7] presented a retrospective evaluation of 658 AF patients with a high fall risk assessed using the Northern Hospital modified STRATIFY tool [21][22]. The overall median age of patients was 75 [31–96] years, which was comparable for all three DOACs (78 years for apixaban, 72 years for rivaroxaban, and 76 years for dabigatran etexilate), with an even distribution by sex One of the study endpoints was clinically significant bleeding that scored three or more points on the 2010 ISTH (International Society of Thrombosis and Haemostasis) bleeding scale [23]. The analysis showed that a high fall risk constitutes a risk factor for clinically significant bleeding (p = 0.032).
The present study examined several parameters that determine the risk of falls: the Timed Up and Go Test, falls in the previous year, and fall-related injuries on the Age is No barrier scale. The study found that none of these parameters had a significant effect on the risk of developing CRNMB. The difference in results can be attributed to the study design, the difference in the age of the examined patients, and the older age of the patients (Me 84 [ 82; 88] years vs Me 75 [ 31; 96] years). Another reason is the difference in the criteria for the definition of bleeding: this study used the 2015 ISTH definition of CRNMB, whereas the article discussed above studied clinically significant bleeding according to the 2010 ISTH scale, which required medical intervention and the use of tranexamic acid, surgical hemostasis, transfusion, infusion of clotting factor concentrates and desmopressin, i.e., major bleeding as defined by the 2015 ISTH scale.
A number of studies examined the effect of frailty on the risk of bleeding. The retrospective study by Yamamoto et al. [8] included 240 AF patients (mean age of 76.1 ± 10.0 years) on DOACs, with women accounting for 42.9%. The patients were divided into frail (5–9 points) or fit (1–4 points) as per the 2005 Rockwood scale [24]. Frail patients accounted for 50% (mean age of 81.2 ± 7.8 years) of the total number of patients; women, 55.8%. The study endpoint was the cumulative incidence of major or symptomatic bleeding in a critical area or organ according to the definitions established by the 2005 ISTH [25]. The study results showed that the cumulative incidence of bleeding was significantly higher in the frail patient group as compared to the fit patients (p < 0.001).
In [9], Søgaard et al. present the results of an observational cohort study examining the safety of DOACs as compared to warfarin in frail AF patients. The study included 32,048 patients who had not previously taken anticoagulants. The median age of patients receiving a standard DOAC dose was 75 (69; 81) years. Patient frailty was defined using the hospital frailty risk score (HFRS) [26], with all patients categorized as having “moderate” (5–15 points) or “high” (>15 points) frailty risk. Moderate frailty risk was exhibited by 87.7% of patients (14,138/16,122) receiving a standard DOAC dose, with 12.3% of patients (1984/16,122) having a high risk of frailty. With moderate frailty risk, major bleeding developed in 2.77% of patients (392/14,138) on a standard DOAC dose, and the relative risk of developing major bleeding on a standard DOAC dose amounted to 0.71 (95% CI 0.60–0.84). In the high frailty risk group, major bleeding developed in 3.53% (70/1984) of patients on a standard DOAC dose, and the relative risk for major bleeding was 0.62 (95% CI 0.41 to 0.92). A supporting analysis covering any bleeding events (including CRNMB and minor bleeding) leading to hospitalization showed comparable results. Although the incidence of major bleeding was slightly higher in the group of patients exhibiting high frailty risk than in the moderate frailty risk group, the relative risk of major bleeding was lower in the high frailty risk group.
The study by Candeloro et al. [10] reports on the association of frailty levels in AF patients with a higher risk of such adverse clinical events as clinically significant bleeding, including major bleeding and CRNMB. The patients were grouped as frail, pre-frail, and fit. Among the 236 patients included in the study (median age of 78 years, with women accounting for 44%), 156 (66%) patients had AF, and 80 (34%) patients had venous thromboembolism. Ninety-eight patients (41%) were frail, 115 patients (49%) were pre-frail, and 23 patients (10%) were fit. During a median observation period of 304 days, the incidence of clinically significant bleeding amounted to 20% in frail patients and 10% in pre-frail patients; no clinically significant bleeding occurred in fit patients.
In [11], Kim et al. present the results of a retrospective study on the effect of frailty on the relationship between DOACs (dabigatran, rivaroxaban, and apixaban) and major bleeding in AF patients as compared to warfarin. Frailty was determined using a deficit accumulation model (claims-based frailty index) [27]. The patients were divided into the following groups: fit at CFI < 0.15, pre-frail at CFI of 0.15–0.24, and frail at CFI ≥ 0.25. The mean age of patients was 76.4 ± 7.1 years in the dabigatran-warfarin cohort, 76.8 ± 7.3 years in the rivaroxaban-warfarin cohort, and 77.3 ± 7.4 years in the apixaban-warfarin cohort. The results of this study are presented as cases per 1000 person-years. The study results showed that the incidence of major, gastrointestinal, and intracranial bleeding increased with increasing frailty.
Unlike the studies described above, the present study assessed the frailty of patients using several scales: Age is No Barrier, Short Physical Performance Battery, 2010 Rockwood frailty index, and the SPRINT scale. It was shown that frailty, defined by these parameters, had no significant effect on the risk of developing CRNMB; in addition, the CRNMB patients were more fit according to the SPRINT frailty index. The difference in results can also be attributed to the study design, the age of patients, the criteria for the definition of bleeding, and the method for measuring target parameters.
CONCLUSION
This study revealed that socially active, emotionally stable, mobile, and fit patients have a higher risk of developing CRNMB. Conversely, frail patients may be less likely to experience CRNMB. Also, a lower incidence of CRNMB was linked to reduced social activity, depression, need for assistance, and use of assistive devices. The study identified geriatric factors that may serve as predictors of CRNMB in AF patients aged over 80 years on DOACs, which were used to develop a prognostic nomogram that can be used to estimate the probability of CRNMB. Significant prognostic factors included the number of household members, Barthel ADL score, Lawton IADL score, Timed Up and Go Test, and use of assistive devices. Given the active AI implementation in clinical practice, the obtained data can be integrated into clinical decision support systems. This would provide a means to assess the risk of developing CRNMB prior to its onset and select high-risk patients to continue work on the prevention of this adverse event.
1. Sychev D.A., Tkacheva O.N., Kotovskaya Yu.V., Malaya I.P. Pharmacotherapy in the aged and elderly. Moscow: KONGRESSKHIM; 2024. (In Russ.)
2. Ministry of Health of the Russian Federation. Russian Association of Gerontologists and Geriatricians. Clinical Guidelines “Senile Frailty.” 2024. Available: https://rgnkc.ru/images/metod_materials/%D0%9A%D0%A0613.pdf
3. Ministry of Health of the Russian Federation. Order No. 38n as of January 29, 2016 “On Approval of the Geriatric Medical Care Protocol (as amended on by the orders of the Ministry of Health of the Russian Federation No. 1067n as of December 20, 2019, No. 114n as of February 21, 2020, and No. 148n as of March 29, 2024)
4. Ministry of Health of the Russian Federation. Russian Association of Gerontologists and Geriatricians. Clinical Guidelines “Senile Frailty.” 2024. Available: https://rgnkc.ru/images/metod_materials/%D0%9A%D0%A0613.pdf
5. Russian Association of Gerontologists and Geriatricians. Patient chart: comprehensive geriatric assessment. Available: https://rgnkc.ru/images/metod_materials/Pacient_card.pdf
6. Ministry of Health of the Russian Federation. Russian Association of Gerontologists and Geriatricians. Clinical Guidelines “Senile Frailty.” 2024. Available: https://rgnkc.ru/images/metod_materials/%D0%9A%D0%A0613.pdf
7. Ministry of Health of the Russian Federation. Russian Association of Gerontologists and Geriatricians. Russian Society of Psychiatrists. Cognitive impairment in the aged and elderly. 2020. Available: https://rgnkc.ru/images/metod_materials/KR_KR.pdf
References
1. Arakelyan MG, Bockeria LA, Vasilieva EYu, Golitsyn SP, Golukhova EZ, Gorev MV, Davtyan KV, Drapkina OM, Kropacheva ES, Kuchinskaya EA, Lajovich LYu, Mironov NYu, Mishina IE, Panchenko EP, Revishvili ASh, Rzayev FG, Tatarsky BA, Utsumueva MD, Shakhmatova OO, Shlevkov NB, Shpektor AV, Andreev DA, Artyukhina EA, Barbarash OL, Galyavich AS, Duplyakov DV, Zenin SA, Lebedev DS, Mikhailov EN, Novikova NA, Popov SV, Filatov AG, Shlyakhto EV, Shubik YuV. 2020 Clinical guidelines for Atrial fibrillation and atrial flutter. Russian Journal of Cardiology. 2021;26(7):4594 (In Russ.). https://doi.org/10.15829/1560-4071-2021-4594
2. Van Gelder IC, Rienstra M, Bunting KV, Casado-Arroyo R, Caso V, Crijns HJGM, De Potter TJR, Dwight J, Guasti L, Hanke T, Jaarsma T, Lettino M, Løchen ML, Lumbers RT, Maesen B, Mølgaard I, Rosano GMC, Sanders P, Schnabel RB, Suwalski P, Svennberg E, Tamargo J, Tica O, Traykov V, Tzeis S, Kotecha D; ESC Scientific Document Group. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45(36):3314–3414. https://doi.org/10.1093/eurheartj/ehae176
3. Tkacheva ON, Vorobyeva NM, Kotovskaya YuV, Runikhina NK, Strazhesco ID, Villevalde SV, Drapkina OM, Komarov AL, Orlova YaA, Panchenko EP, Pogosova NV, Frolova EV, Yavelov IS. Antithrombotic therapy in the elderly and senile age: the consensus opinion of experts of the Russian Association of Gerontologists and Geriatricians and the National Society of Preventive Cardiology. Cardiovascular Therapy and Prevention. 2021;20(3):2847 (In Russ.). https://doi.org/10.15829/1728-8800-2021-2847
4. Zagidullin NSh, Davtyan PA. Specifics of anticoagulation in combination with atrial fibrillation and chronic kidney disease. Russian Journal of Cardiology. 2021;26(3S):4676 (In Russ.). https://doi.org/10.15829/1560-4071-2021-4676
5. Filonova UD, Karnakova PK, Karnakova KK, Popova MO, Popova AA, Archakova OA, Komarov TN, Shohin .E. Development and Validation of an HPLC-MS/MS Method for Quantification of Apixaban in Human Plasma. Drug development & registration. 2024;13(1):224–240. https://doi.org/10.33380/2305-2066-2024-13-1-1684
6. Hindricks G, Potpara T, Dagres N, Arbelo Е, Bax JJ, Blomstrӧm-Lundqvist С, Boriani G, Castella M, Dan G, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J, Lettino M, Lip GY, Pinto FJ, Neil Thomas G, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL. Рекомендации ESC 2020 по диагностике и лечению пациентов с фибрилляцией предсердий, разработанные совместно с европейской ассоциацией кардиоторакальной хирургии (EACTS). Российский кардиологический журнал. 2021;26(9):4701. https://doi.org/10.15829/1560-4071-2021-4701
7. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrӧm-Lundqvist C, Boriani G, Castella M, Dan G, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J, Lettino M, Lip GY, Pinto FJ, Neil Thomas G, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Russian Journal of Cardiology. 2021;26(9):4701 (In Russ.). https://doi.org/10.15829/1560-4071-2021-4701
8. Brook R, Aswapanyawongse O, Tacey M, Kitipornchai T, Ho P, Lim HY. Real-world direct oral anticoagulant experience in atrial fibrillation: falls risk and low dose anticoagulation are predictive of both bleeding and stroke risk. Intern Med J. 2020;50(11):1359–1366. h ttps://doi.org/10.1111/imj.14640
9. Yamamoto T, Yamashita K, Miyamae K, Koyama Y, Izumimoto M, Kamimura Y, Hayakawa S, Mori K, Yamada T, Tomita Y, Murohara T. The influence of frailty under direct oral anticoagulant use in patients with atrial fibrillation. Heart Asia. 2019;11(2):e011212. https://doi.org/10.1136/heartasia-2019-011212
10. Søgaard M, Ording AG, Skjøth F, Larsen TB, Nielsen PB. Effectiveness and safety of direct oral anticoagulation vs. warfarin in frail patients with atrial fibrillation. Eur Heart J Cardiovasc Pharmacother. 2024;10(2):137–146. https://doi.org/10.1093/ehjcvp/pvad091
11. Candeloro M, Di Nisio M, Potere N, Di Pizio L, Secinaro E, De Flaviis C, Federici C, Guglielmi MD, Pardi S, Schulman S, Porreca E. Frailty phenotype as a predictor of bleeding and mortality in ambulatory patients receiving direct oral anticoagulants. J Am Geriatr Soc. 2022;70(12):3503–3512. https://doi.org/10.1111/jgs.18001
12. Kim DH, Pawar A, Gagne JJ, Bessette LG, Lee H, Glynn RJ, Schneeweiss S. Frailty and Clinical Outcomes of Direct Oral Anticoagulants Versus Warfarin in Older Adults With Atrial Fibrillation: A Cohort Study. Ann Intern Med. 2021;174(9):1214–1223. https://doi.org/10.7326/M20-7141
13. Сычев Д.А., Черняева М.С., Рожкова М.А., Моисеева Е.А., Погодина А.А., Байзель Ю.С., Егорова Л.А., Масленникова О.М., Ломакин Н.В. Безопасность прямых оральных антикоагулянтов в лечении фибрилляции предсердий у гериатрических пациентов: фокус на клинически значимые небольшие кровотечения. Фарматека. 2024;31(4):8–23. https://doi.org/10.18565/pharmateca.2024.4.8-23
14. Sychev DA, Cherniaeva MS, Rozhkova MA, Moiseeva A., Pogodina AA, Bayzel YuS, Egorova LA, Maslennikova OM, Lomakin NV. Safety of direct oral anticoagulants in the treatment of atrial fibrillation in geriatric patients: focus on clinically relevant non-major bleeding. Farmateka. 2024;31(4):8–23 (In Russ.). https://doi.org/10.18565/pharmateca.2024.4.8-23
15. Cherniaeva MS, Rozhkova MA, Kondrakhin AP, Pogodina AA, Makhmudov IN, Alisultanov DA, Egorova LA, Maslennikova OM, Lomakin NV, Sychev DA. Frequency of occurrence and structure of clinically significant minor bleedings against the background of taking direct oral anticoagulants in patients 80 years and older with nonvalvular atrial fibrillation. Effective Pharmacotherapy. 2024;20(26):28– 38 (In Russ.). https://doi.org/10.33978/2307-3586-2024-20-26-28-38
16. Bonanad C, Formiga F, Anguita M, Petidier R, Gullón A. Oral Anticoagulant Use and Appropriateness in Elderly Patients with Atrial Fibrillation in Complex Clinical Conditions: ACONVENIENCE Study. J Clin Med. 2022;11(24):7423. https://doi.org/10.3390/jcm11247423
17. Mailhot T, McManus DD, Waring ME, Lessard D, Goldberg R, Bamgbade BA, Saczynski JS. Frailty, Cognitive Impairment, and Anticoagulation Among Older Adults with Nonvalvular Atrial Fibrillation. J Am Geriatr Soc. 2020;68(12):2778–2786. https://doi.org/10.1111/jgs.16756
18. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65.
19. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179– 186.
20. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony P, Charlton KE, Maggio M, Tsai AC, Grathwohl D, Vellas B, Sieber CC; MNA-International Group. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782–788. https://doi.org/10.1007/s12603-009-0214-7
21. Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc. 2010;58(2):318–323. https://doi.org/10.1111/j.1532-5415.2009.02667.x
22. Pajewski NM, Williamson JD, Applegate WB, et al. Characterizing Frailty Status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci. 2016;71(5):649–655. https://doi.org/10.1093/gerona/glv228
23. Cherniaeva MS, Alferova PA, Pavlova AO, Trifonov MI, Egorova LA, Maslennikova OM. Lomakin NV, Sychev DA. Safety of direct oral anticoagulants in patients with atrial fibrillation aged 80 years and older: clinical risk factors. Russian Medicine. 2024;30(5):464–474 (In Russ.). https://doi.org/10.17816/medjrf635877
24. Barker A, Kamar J, Graco M, Lawlor V, Hill K. Adding value to the STRATIFY falls risk assessment in acute hospitals. J Adv Nurs. 2011;67(2):450–457. https://doi.org/10.1111/j.1365-2648.2010.05503.x
25. Rodeghiero F, Tosetto A, Abshire T, Arnold DM, Coller B, James P, Neunert C, Lillicrap D; ISTH/SSC joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders.. J Thromb Haemost. 2010;8(9):2063–2065. https://doi.org/10.1111/j.1538-7836.2010.03975.x
26. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. https://doi.org/10.1503/cmaj.050051
27. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in nonsurgical patients. J Thromb Haemost. 2005;3(4):692–694. https://doi.org/10.1111/j.1538-7836.2005.01204.x
28. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, Bardsley M, Conroy S. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–1782. https://doi.org/10.1016/S0140-6736(18)30668-8
29. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. https://doi.org/10.1093/gerona/glx229
About the Authors
M. S. CherniaevaРоссия
Marina S. Cherniaeva — Cand. Sci. (Med.), Assoc. Prof., Department of Clinical Pharmacology and Therapy named after Academician B.E. Votchal; Assoc. Prof., Department of Internal and Preventive Medicine; geriatrician, Head of the Geriatric Department No. 5
Barrikadnaya str., 2/1, bldg. 1, Moscow, 125993
Marshala Timoshenko str., 19, bldg. 1a, Moscow, 121359
Volgogradsky Ave., 168, Moscow, 109472
A. O. Pavlova
Россия
Anna O. Pavlova — Assistant, Department of Internal and Preventive Medicine
Marshala Timoshenko str., 19, bldg. 1a, Moscow, 121359
P. A. Alferova
Россия
Polina A. Alferova — Assistant, Department of Clinical Pharmacology and Therapy named after Academician B.E. Votchal
Barrikadnaya str., 2/1, bldg. 1, Moscow, 125993
M. A. Rozhkova
Россия
Maria A. Rozhkova — primary care physician
Orekhovyi Blvd., 28, Moscow, 115682
M. I. Trifonov
Россия
Mikhail I. Trifonov — geriatrician
3rd Monetchikovsky Ln., 16, bldg. 1, Moscow, 115054
T. V. Nikeeva
Россия
Tatyana V. Nikeeva — primary care physician, Consulting Department
Volgogradsky Ave., 168, Moscow, 109472
L. A. Egorova
Россия
Larisa A. Egorova — Dr. Sci. (Med.), Prof., Department of Internal and Preventive Medicine
Marshala Timoshenko str., 19, bldg. 1a, Moscow, 121359
O. M. Maslennikova
Россия
Olga M. Maslennikova — Dr. Sci. (Med.), Assoc. Prof., Head of
the Department of Internal and Preventive Medicine
Marshala Timoshenko str., 19, bldg. 1a, Moscow, 121359
N. V. Lomakin
Россия
Nikita V. Lomakin — Dr. Sci. (Med.), Assoc. Prof., Head of the Cardiology Department; Head of the Cardiology Department No. 2
Marshala Timoshenko str., 15, Moscow, 121359
D. A. Sychev
Россия
Dmitry A. Sychev — Dr. Sci. (Med.), Prof., Prof. of the Russian Academy of Sciences, Academician of the Russian Academy of Sciences, Head of the Department of Clinical Pharmacology and Therapy named after Academician B.E. Votchal
Marshala Timoshenko str., 15, Moscow, 121359
Supplementary files
Review
For citations:
Cherniaeva M.S., Pavlova A.O., Alferova P.A., Rozhkova M.A., Trifonov M.I., Nikeeva T.V., Egorova L.A., Maslennikova O.M., Lomakin N.V., Sychev D.A. Geriatric factors affecting the safety of direct oral anticoagulants in atrial fibrillation patients aged 80 years and older: An observational prospective cohort study. Kuban Scientific Medical Bulletin. 2025;32(3):15-35. https://doi.org/10.25207/1608-6228-2025-32-3-15-35
JATS XML








































