Scroll to:
Physical development of preterm monochorionic diamniotic twins at birth: retrospective cohort study
https://doi.org/10.25207/1608-6228-2023-30-1-37-48
Abstract
Background. Much controversy surrounds the estimation of anthropometric parameters in multiple newborns. In newborn monochorionic diamniotic twins, these parameters can be affected by specific antenatal complications.
Objectives. To estimate the physical development parameters of preterm monochorionic diamniotic twins according to standards proposed within the INTERGROWTH-21st project, taking specific intrauterine complications into account.
Methods. The anthropometric data were analyzed in 148 pairs of newborn monochorionic diamniotic twins, who were divided into three groups according to the presence of specific intrauterine complications: Group I (n = 56 pairs) — twin-to-twin transfusion syndrome (TTTS); Group II (n = 38 pairs) — selective intrauterine growth restriction (sIUGR); Group III (n = 58 pairs) — absence of the above-mentioned specific complications. The obtained data were statistically processed on a personal computer via variation statistics methods using Microsoft Excel spreadsheets (Microsoft, USA) and an online service available at https://medstatistic.ru.
Results. Newborns with sIUGR (37–100.0%) and 26 (49.1%) donors fell into the category of newborns light for gestational age. Disharmonious (3–10th percentile) and markedly disharmonious (below the 3rd percentile) physical development at birth was most commonly observed in newborns with sIUGR and, to a lesser extent, in donors (OR — 9.2; 95% CI — 3.2–24.3; p < 0.05), which was noted only occasionally in monochorionic twins from other groups. A combined decrease in the values of birth centiles for head circumference, body weight, and body length was found in 13 (35.1%) newborns with sIUGR and in 12 (22.6%) donors, which may mark the severity of antenatal complications and the development of neurological deficit.
Conclusion. Monochorionic diamniotic twins include newborns having greater and lower body weights. In the newborn having a lower birth weight, specific complications associated with monochorionic multiple pregnancy result in body weight and length deficit, disharmonious development due to the lack of nourishment (22.6% in the TTTS group and 73.0% in the sIUGR group), as well as delayed head circumference growth in 56.8% of newborns with sIUGR.
Keywords
For citations:
Pavlichenko M.V., Kosovtsova N.V., Pospelova Ya.Yu., Markova T.V. Physical development of preterm monochorionic diamniotic twins at birth: retrospective cohort study. Kuban Scientific Medical Bulletin. 2023;30(1):37-48. https://doi.org/10.25207/1608-6228-2023-30-1-37-48
INTRODUCTION
The physical development of newborn babies is fundamental in characterizing their state of health. Anthropometric data are commonly obtained in the first minutes of life following Apgar score calculation, which have high clinical and diagnostic significance. The parameters of physical development are thought to be predetermined by inherited genetic information and realized depending on performance under ambient conditions [1][2]. Intrauterine growth constitutes an essential stage affecting the variable growth process and the morphofunctional maturation of fetal organs and systems [3][4]. To date, much controversy surrounds the estimation of anthropometric parameters in multiple newborns, specifically in monochorionic placentation, due to specific complications arising in the antenatal period: twin-to-twin transfusion syndrome (TTTS) and selective intrauterine growth restriction (sIUGR) [5][6]. Intrauterine complications leading to changes in the anthropometric data of newborns largely determine their future health status and quality of life1 [7][8]. In routine clinical practice, neonatologists can use the INTERGROWTH-21st project to assess the physical development of newborns. Since changes in the physical development parameters of preterm neonates are associated with a high risk of maladaptation, as well as neonatal incidence and mortality [9][10], a study was conducted in order to determine the potential of specific intrauterine complications in predicting the physical development of preterm monochorionic diamniotic twins [13][14].
Taking specific intrauterine complications into account, the study aims to estimate the physical development parameters of preterm monochorionic diamniotic twins according to standards proposed within the INTERGROWTH-21st project.
METHODS
Study design
A cross-sectional, retrospective cohort study was conducted.
Study conditions
The study was conducted at the Ural Research Institute of Maternal and Infant Care (Ministry of Health of the Russian Federation), analyzing the medical records of newborn monochorionic diamniotic twins delivered there in 2018–2021.
Eligibility criteria
Inclusion criteria
Preterm monochorionic diamniotic twins.
Exclusion criteria
Full-term monochorionic diamniotic twins, newborns in singleton pregnancies, newborn dichorionic twins, and twin reversed arterial perfusion sequence.
Removal criteria
Patients whose medical records lacked information necessary for the analysis.
Description of eligibility criteria (diagnostic criteria)
Birth anthropometric data documented in medical records were entered into the study database: body weight, body length, head circumference, as well as sex and gestational age of patients.
Selection of group members
The main cohort was retrospectively divided into three groups depending on the presence of specific intrauterine complications: twin-to-twin transfusion syndrome (TTTS), selective intrauterine growth restriction (sIUGR), and the absence of the specified complications associated with monochorionic multiple pregnancies. Group I comprises 56 pairs of monochorionic twins treated for TTTS using the laser coagulation of placental anastomoses at a gestational age of 16–21 weeks, whereas Group II consists of 38 monochorionic twin pairs with sIUGR in one of the fetuses: birth weight values are found within the extremely low (P < 3), or low (P < 10) centile band. Group III includes 56 pairs of monochorionic twins with no antenatal complications (TTTS and sIUGR).
Target parameters in the study
Main parameter in the study
The main birth parameters of physical development were estimated according to the INTERGROWTH-21st project: weight (kg), length (cm), head circumference (cm), and weight-length ratio (kg/m), indicating harmonious physical development.
Additional parameters in the study
Sex and gestational age depending on the presence or absence of specific complications: TTTS, sIUGR, and the absence of the specified complications.
Methods for measuring the parameters
Each patient was examined for body weight, body length, head circumference, and weight-length ratio under the INTERGROWTH-21st project; sex and gestational age of preterm monochorionic diamniotic twins, as well as the presence/absence of specific antenatal complications, were obtained from medical records.
Variables (predictors, confounders, and effect modifiers)
The birth parameters of physical development covered by the INTERGROWTH-21st project.
Statistical procedures
Principles behind sample size determination
The sample size was not determined in advance.
Statistical methods
The obtained data were statistically processed on a personal computer via variation statistics methods using Microsoft Excel spreadsheets (Microsoft, USA) and an online service available at https://medstatistic.ru. Qualitative parameters were described in terms of the absolute and relative frequencies of their values. The distribution of the examined anthropometric data differs from the normal distribution. The results are presented as a median of the interquartile range [ 25th percentile; 75th percentile], odds ratio (OR), and a 95% confidence interval (CI). The equality of medians was checked for several samples using the non-parametric Kruskal-Wallis test designed for independent populations. Differences were considered to be statistically significant at p < 0.05 and P < 0.01.
RESULTS
Sampling
According to the protocol, 148 pairs of preterm monochorionic twins (296 patients) were initially included in the study; however, the final analysis included the parameters of 292 newborns (Fig. 1).
Characteristics of the study sample
According to the obtained data, intrauterine fetal death was observed in two recipient fetuses, three donor fetuses, and one fetus exhibiting sIUGR signs. The overall mortality rate in the MDT group amounted to 2.03%, including 4.5% in Group I and 1.3% in Group II.
Main study results
The target parameters were estimated in relation to the following aspects: donor/recipient; newborns with/without sIUGR; twin having a greater/lower birth weight. In addition, Groups I, II, and III were compared in terms of body weight: a donor from Group I — a patient with sIUGR from Group II — a twin having a lower birth weight from Group III; a recipient from Group I — a twin without sIUGR from Group II — a newborn having a greater birth weight from Group III.
For all the examined groups, highly significant body weight differences were revealed in the subgroups of monochorionic twins (p < 0.001). In Group II, sIUGR was observed in both fetuses in three pairs (7.9%) at a gestational age of 33–34 weeks. All newborns with sIUGR had a birthweight of <10th centile, which was significantly (p < 0.001) lower than that of Group III twins having a lower body weight. In 21 donors (39.6%), body weight values were also found within a low centile band of P3- <10; no significant differences in this parameter were revealed from newborns with sIUGR.
The body weight of newborns indicates the development of the musculoskeletal system, subcutaneous tissue, and internal organs; the estimation of this parameter according to centile bands under the INTERGROWTH-21st project provides neonatologists with meaningful additional information about the antenatal period [15][16].
Birth weight values were found within the 10–90 centile band for the majority of recipients, Group III patients, and Group II newborns exhibiting no signs of sIUGR.
As well as birth weight, body length constitutes a fundamental criterion in the comprehensive health assessment of newborns.
Highly significant body length differences were revealed between the monochorionic twin subgroups in Groups I, II, and III (p < 0.001). All newborns with sIUGR exhibited symmetrical growth restrictions (body weight and length), as well as falling into the category of newborns light for gestational age (ICD-10 code — P05.0). These facts are consistent with the literature on the occurrence of fetal complications in newborns with sIUGR in early pregnancy, commonly during the first trimester [17][18]. In the donor group treated for TTTS, 26 (49.1%) neonates were also classified as newborns light for gestational age, which may result from the early onset of the transfusion syndrome. Only one recipient (1.9%) was found to be a newborn light for gestational age (ICD-10 code — P05.0), while other groups exhibited no such signs.
The harmonicity of physical development in newborns was estimated according to weight-height charts [19].
Harmonious development was observed in the majority of monochorionic twins from all groups, with the exception of newborns exhibiting the signs of sIUGR. Disharmonious and markedly disharmonious development was most common in newborns with sIUGR and, to a lesser extent, in donors (OR — 9.2; 95% CI — 3.2–24.3; p < 0.05); in monochorionic twins from other groups, it occurred in individual cases.
The analysis of head circumference also revealed significant differences between examined monochorionic twins.
Highly significant head circumference differences were revealed between the monochorionic twin subgroups in Groups I, II, and III (p < 0.001). In the group of neonates treated for TTTS, head circumference was found to be within the normal interval (p10–90) in 41 recipients (75.9%) and 38 donors (71.7%). In Group II, patients with sIUGR were significantly more likely to have a head circumference of P < 10 (OR –3.7; 95% CI — 1.4–9.7; p < 0.05): in 21 patients with sIUGR (56.8%) and 10 newborns (26.3%) without sIUGR. In Group III, all patients predominantly exhibited a range of normal head circumference values: in the monochorionic twins of greater weight — 50 (86.2%); in the monochorionic twins of lower weight — 53 (91.4%).
A physiological increase in head circumference during the antenatal period indicates active cell differentiation in the central nervous system, which is of fundamental importance for predicting cognitive development in infancy and early childhood [20][21]. In the event that intrauterine growth restriction is systemic in nature and does not correspond to the inherent genetic program for organism development, the long-term rehabilitation prognosis may be poor [20].
A symmetrical decrease in the values of birth centiles for head circumference, body weight, and body length was found in Groups I and II: in 13 (35.1%) newborns with sIUGR as compared to 1 MDT (2.6%) without sIUGR (OR — 20.0; 95% CI — 2.46–163.3; p < 0.05); in 12 donors (22.6%) as compared to 1 (1.9%) recipient (OR — 15.5; 95% CI — 1.94–124.2; p < 0.05). Group III revealed no newborns with similar changes. The delayed growth rate of head circumference may subsequently contribute to the development of neurological deficit, requiring prolonged dynamic monitoring2 3 [21][22].
Additional study parameters
The distribution of patients by gestational age is shown in Table 5.
The gestational age of monochorionic twins in uncomplicated pregnancies was significantly higher than that in the group of newborns treated for TTTS (p < 0.001) and with sIUGR (p = 0.01). Extremely preterm newborns (less than 28 weeks of gestation) were noted only in the group of monochorionic twins suffering from specific antenatal complications. No significant differences were observed between the groups in the number of very preterm neonates (28 weeks 0 days to 31 weeks 6 days). Births were moderately preterm for 42.9% of newborns treated for TTTS (OR — 4.6; 95% CI — 2.05–10.41; p < 0.05) and for 57.9% of neonates with sIUGR (OR — 2.52; 95% CI — 1.03–6.14; p < 0.05) as compared to 77.6% of Group III neonates.
Boys accounted for 26 pairs (46.4%) in the group of newborns treated for TTTS, 23 pairs (60.5%) in the group with sIUGR, and 30 pairs (51.7%) in the group suffering from no antenatal complications; thus, no significant sex-specific differences were revealed.
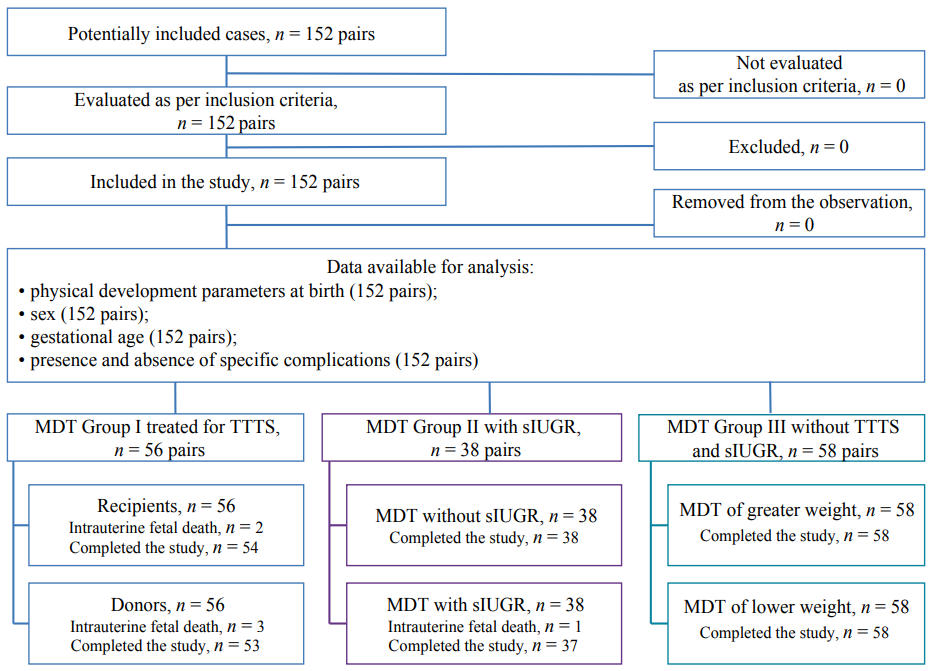
Fig. 1. Study design.
Note: A flow-chart diagram completed by the authors according to the STROBE recommendations; MDT — monochorionic diamniotic twins; TTTS — twin-to-twin transfusion syndrome; sIUGR — selective intrauterine growth restriction.
Рис. 1. Дизайн исследования.
Примечание: Блок-схема согласно рекомендациям STROBE заполнена авторами; МХБ — монохориальные диамниотические близнезы; СФФТ — синдром фето-фетальной трансфузии; ССЗПР — синдром селективной задержки роста одного из плодов.
Table 1. Birth body weight parameters in monochorionic diamniotic twins
Таблица 1. Оценка показателей массы тела при рождении у близнецов из монохориальных диамниотических двоен
|
No. |
Patients |
Weight, g Ме [Q25%; Q75%] |
Intra-group significance of differences, р |
Intergroup |
|
|
Group I — TTTS |
1:3 = 0.99 1c:3c < 0.001 1:5 < 0.001 1c:5c = 0.08 2:4 = 0.44 2c:4c = 0.04 2:6 < 0.001 2c:6c = 0.03 3:5 < 0.001 3c:5c < 0.001 4:6 = 0.001 4c:6c = 0.81 |
||
|
1 |
donor, n = 53 |
1260.0 [ 780.0; 1680.0] |
< 0.001 |
|
|
1c |
centile |
15.58 [ 2.66; 36.05] |
< 0.001 |
|
|
2 |
recipient, n = 54 |
1595.0 [ 1175.0; 2015.0] |
|
|
|
2c |
centile |
53.59 [ 40.14; 73.48] |
|
|
|
|
Group II — sIUGR |
|||
|
3 |
twin with sIUGR, n = 37 |
1140.0 [ 800.0; 1570.0] |
< 0.001 |
|
|
3c |
centile |
2.81 [ 0.62; 4.45] |
< 0.001 |
|
|
4 |
twin without sIUGR, n = 38 |
1555.0 [ 12550.0; 1990.0] |
|
|
|
4c |
centile |
40.30 [ 20.3; 53.29] |
|
|
|
|
Group III — without TTTS and sIUGR |
|||
|
5 |
twin of lower weight, n = 50 |
1769.0 [ 1560.0; 1970.0] |
< 0.001 |
|
|
5c |
centile |
29.24 [ 17.90; 42.09] |
< 0.001 |
|
|
6 |
twin of greater weight, n = 50 |
1995.0 [ 1810.0; 2220.0] |
|
|
|
6c |
centile |
50.63 [ 28.27; 62.99] |
|
|
Note: the table was compiled by the authors; TTTS — twin-to-twin transfusion syndrome; sIUGR — selective intrauterine growth restriction.
Примечание: таблица составлена авторами; СФФТ — синдром фето-фетальной трансфузии; ССЗПР — синдром селективной задержки роста одного из плодов.
Table 2. Birth body length parameters in monochorionic diamniotic twins
Таблица 2. Оценка показателей длины тела при рождении у близнецов из монохориальных диамниотических двоен
|
№ |
Patients |
Weight, g Ме [Q25%; Q75%] |
Intra-group significance of differences, р |
Intergroup significance of differences, р |
|
|
Group I — TTTS |
1:3 < 0.001 1c:3c < 0.001 1:5 < 0.001 1c:5c = 0.41 2:4 < 0.001 2c:4c < 0.001 2:6 < 0.001 2c:6c = 0.74 3:5 < 0.001 3c:5c < 0.001 4:6 < 0.001 4c:6c = 0.003 |
||
|
1 |
donor, n = 53 |
37.0 [ 32.0; 41.0] |
< 0.001 |
|
|
1c |
centile |
10.0 [ 0.7; 25.0] |
< 0.001 |
|
|
2 |
recipient, n = 54 |
40.0 [ 37.0; 43.0] |
|
|
|
2c |
centile |
30.7 [ 13.7; 56.1] |
|
|
|
|
Group II — sIUGR |
|||
|
3 |
twin with sIUGR, n = 37 |
36.5 [ 32.8; 41.0] |
< 0.001 |
|
|
3c |
centile |
1.4 [ 0.4; 8.4] |
< 0.001 |
|
|
4 |
twin without sIUGR, n = 38 |
41.0 [ 37.0; 43.0] |
|
|
|
4c |
centile |
25.9 [ 11.4; 42.3] |
|
|
|
|
Group III — without TTTS and sIUGR |
|||
|
5 |
twin of lower weight, n = 58 |
41.0 [ 40.0; 43.0] |
< 0.001 |
|
|
5c |
centile |
13.1 [ 6.0; 35.3] |
< 0.001 |
|
|
6 |
twin of greater weight, n = 58 |
43 [ 41.0; 44.8] |
|
|
|
6c |
centile |
37.9 [ 18.3; 54.4] |
|
|
Note: the table was compiled by the authors; TTTS — twin-to-twin transfusion syndrome; sIUGR — selective intrauterine growth restriction.
Примечание: таблица составлена авторами; СФФТ — синдром фето-фетальной трансфузии; ССЗПР — синдром селективной задержки роста одного из плодов.
Table 3. Harmonicity of physical development in newborns according to weight-height charts
Таблица 3. Оценка гармоничности физического развития детей по данным диаграмм соответствия массы тела росту
|
Interval |
Interpretation of the parameter |
Group No. |
Prevalence in newborns, abs. (%) |
|
|
twin of greater weight |
twin of lower weight |
|||
|
Below the 3rd percentile |
Markedly disharmonious development |
I |
0 |
7 (13.2%) |
|
II |
1 (2.6%) |
7 (18.9%) |
||
|
III |
0 |
0 |
||
|
3–10th percentile |
Disharmonious development |
I |
1 (1.9%) |
5 (9.4%) |
|
II |
3 (7.9%) |
20 (54.1%) |
||
|
III |
0 |
1 (1.7%) |
||
|
10–90th percentile |
Harmonious development |
I |
53 (98.1%) |
41 (77.4%) |
|
II |
33 (86.8%) |
10 (27.0%) |
||
|
III |
53 (91.4%) |
54 (93.1%) |
||
|
90–97th percentile |
Disharmonious development |
I |
0 |
0 |
|
II |
1 (2.6%) |
0 |
||
|
III |
4 (6.9%) |
1 (1.7%) |
||
|
Above the 97th percentile |
Markedly disharmonious development |
I |
0 |
0 |
|
II |
0 |
0 |
||
|
III |
1 (1.7%) |
2 (3.4%) |
||
Note: the table was compiled by the authors.
Примечание: таблица составлена авторами.
Table 4. Birth head circumference parameters in monochorionic diamniotic twins
Таблица 4. Оценка показателей окружности головы при рождении у близнецов из монохориальных диамниотических двоен
|
No. |
Patients |
Weight, g Ме [Q25%; Q75%] |
Intra-group significance of differences, р |
Intergroup significance of differences, р |
|
|
Group I — TTTS |
1:3 = 0.71 1c:3c = 0.013 1:5 < 0.001 1c:5c = 0.06 2:4 = 0.58 2c:4c = 0.03 2:6 = 0.007 2c:6c = 0.002 3:5 = 0.001 3c:5c < 0.001 4:6 = 0.003 4c:6c = 0.04 |
||
|
1 |
donor, n = 53 |
28.0 [ 24.0; 31.0] |
< 0.001 |
|
|
1c |
centile |
39.9 [ 7.0; 73.9] |
< 0.001 |
|
|
2 |
recipient, n = 54 |
29.5 [ 27.0; 31.8] |
|
|
|
2c |
centile |
70.5 [ 56.5; 85.5] |
|
|
|
|
Group II — sIUGR |
|||
|
3 |
twin with sIUGR, n = 37 |
29.0 [ 24.8; 30.0] |
< 0.001 |
|
|
3c |
centile |
14.5 [ 5.4; 32.0] |
< 0.001 |
|
|
4 |
twin without sIUGR, n = 38 |
30.0 [ 28.3; 31.8] |
|
|
|
4c |
centile |
62.6 [ 21.9; 81.9] |
|
|
|
|
Group III– without TTTS and sIUGR |
|||
|
5 |
twin of lower weight, n = 58 |
30.0 [ 29.0; 32.0] |
< 0.001 |
|
|
5c |
centile |
51.5 [ 35.7; 69.6] |
< 0.001 |
|
|
6 |
twin of greater weight, n = 58 |
31.0 [ 30.0; 32.0] |
|
|
|
6c |
centile |
72.1 [ 51.5; 82.7] |
|
|
Note: the table was compiled by the authors; TTTS — twin-to-twin transfusion syndrome; sIUGR — selective intrauterine growth restriction.
Примечание: таблица составлена авторами; СФФТ — синдром фето-фетальной трансфузии; ССЗПР — синдром селективной задержки роста одного из плодов.
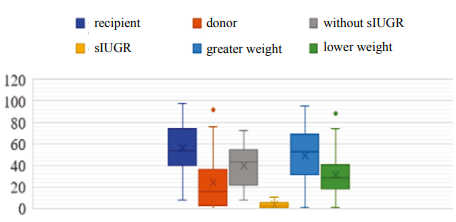
Fig. 2. Body weight centiles in observed newborns under the INTERGROWTH-21st project.
Note: the figure was created by the authors; sIUGR — selective intrauterine growth restriction.
Рис. 2. Центильные величины массы тела наблюдаемых новорожденных по программе «INTERGROWTH-21th».
Примечание: рисунок составлен авторами; ССЗПР — синдром селективной задержки роста одного из плодов.
Table 5. Distribution of newborns by gestational age, taking specific antenatal complications into account
Таблица 5. Распределение детей по гестационному возрасту с учетом наличия специфических осложнений антенатального периода
|
Gestational age (GA) |
Groups |
Significance of differences, р |
|||||
|
I-TTTS (n = 56 pairs) |
II-sIUGR |
III- without TTTS and sIUGR (n = 58 pairs) |
|||||
|
abs. |
% |
abs. |
% |
abs. |
% |
||
|
GA of the group, Ме [Q25%; Q75%], weeks |
31.4 [ 28.0; 33.5] |
32.4 [ 29.1; 34.2]
|
33.2 [ 32.2; 34.2] |
2:3 р > 0.05 2:4 р < 0.001 3:4 р = 0.01 |
|||
|
Less than 27 weeks 6 days |
|||||||
|
Number of newborns, n |
13 |
23.2 |
5 |
13.2 |
0 |
0 |
2:4 p < 0.05 2:3 p > 0.05 3:4 p < 0.05 |
|
Ме [Q25%; Q75%], weeks |
25.4 [ 25.2; 27.0] |
26.0 [ 25.4; 27.2] |
|
2:3 р > 0.05 |
|||
|
28 weeks 0 days — 31 weeks 6 days |
|||||||
|
Number of newborns, n |
19 |
33.9 |
11 |
28.9 |
13 |
22.4 |
2:3 p > 0.05 2:4 p > 0.05 3:4 p > 0.05 |
|
Ме [Q25%; Q75%], weeks |
29.5 [ 28.4; 30.8] |
29.5 [ 28.8; 30.4] |
30.5 [ 29.5; 31.3] |
2:3 р > 0.05 2:4 р > 0.05 3:4 р > 0.05 |
|||
|
Over 32 weeks |
|||||||
|
Number of newborns, n |
24 |
42.9 |
22 |
57.9 |
45 |
77.6 |
2:3 p > 0.05 2:4 p < 0.05 3:4 p < 0.05 |
|
Ме [Q25%; Q75%], weeks |
33.5 [ 32.4; 34.4] |
34.1 [ 33.4; 34.4] |
33.4 [ 32.4; 34.3] |
2:3 р > 0.05 2:4 р > 0.05 3:4 р > 0.05 |
|||
Note: the table was compiled by the authors; GA — gestational age; TTTS — twin-to-twin transfusion syndrome; sIUGR — selective intrauterine growth restriction.
Примечание: таблица составлена авторами; ГВ — гестационный возраст; СФФТ — синдром фето-фетальной трансфузии; ССЗПР — синдром селективной задержки роста одного из плодов.
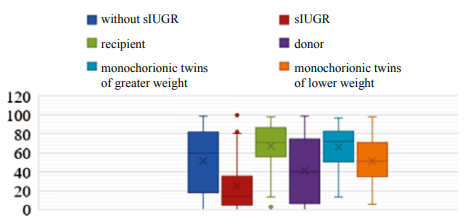
Fig. 3. Head circumference centiles in the examined newborns under the INTERGROWTH-21st project.
Note: the figure was created by the authors; sIUGR — selective intrauterine growth restriction.
Рис. 3. Центильные величины окружности головы наблюдаемых новорожденных по программе «INTERGROWTH-21th».
Примечание: рисунок составлен авторами; ССЗПР — синдром селективной задержки роста одного из плодов.
DISCUSSION
Main findings of the study
The study was aimed at assessing physical development in preterm monochorionic diamniotic twins with and without TTTS and sIUGR under the INTERGROWTH-21st project. The INTERGROWTH-21st project provides a means to comprehensively analyze anthropometric data in preterm monochorionic twins, revealing characteristics associated with antenatal complications.
Research limitations
The study has several limitations. This single-center study conducted in 2018–2021 used a small number of patients, which may affect the final results, thus limiting the potential to extend these findings to a larger sample.
Discussion of the main study findings
The anthropometric data of newborns are used to assess the adequacy of fetal and neonatal growth, predict delayed growth abnormalities, as well as to assess neonatal incidence and mortality. The high rate of perinatal incidence and mortality in monochorionic multiple pregnancies is attributed both to a high frequency of preterm births and, to a large extent, specific complications in monochorionic placentation [11][12]. The obtained data indicate varying physical development disorders in preterm monochorionic twins associated with the complicated antenatal period. The use of international standards governing neonatal growth under INTERGROWTH-21st project enables a more effective assessment of the impact made by specific antenatal complications (TTTS and sIUGR) on the physical development of monochorionic diamniotic twins. The feeding and nursing of preterm monochorionic diamniotic twins classified as newborns light for gestational age, as well as newborns with disharmonious and markedly disharmonious physical development, should be different from that of gestational-age appropriate neonates [23]. In the group of monochorionic diamniotic twins, preterm neonates born following antenatal complications deserve increased attention: donors (TTTS) and patients exhibiting the signs of sIUGR. In order to prevent diseases commonly occurring in preterm newborns characterized by deficient physical development, the daily food ration should be maximally enriched with the permissible amounts of protein and energy per actual body weight.
As newborns’s harmonious development in later years can be largely determined by birth anthropometric parameters [20], it is imperative to prolong the physical development assessment under the INTERGROWTH-21st project in preterm monochorionic twins after antenatal complications, which will provide a means to optimize diagnosis and treatment for this group of patients.
CONCLUSION
Monochorionic diamniotic twin pairs include newborns having a higher and lower body weight. In the newborns of a lower birth weight, specific complications associated with monochorionic multiple pregnancy result in disharmonious development due to the lack of nourishment (22.6% in the TTTS group and 73.0% in the sIUGR group), as well as delayed head circumference growth in 56.8% of neonates with sIUGR.
1 Volodin N.N., Clinical guidelines. Neonatology. Ed. by Volodina N.N., Degtyareva D.N., Kryuchko D.S.; GEOTAR-Media, Moscow, 2019.
2 Akimova E.A. et al., Neurobiological principles underlying the emergence and treatment of the perinatal injury of the central nervous system in newborns. Ed. by Namazova-Baranova L. S., Moscow: Pediatr, 2016.
3 Infant Feeding Optimization Program in the Russian Federation, Guidelines, Moscow, 2019. Available at: https://nczd.ru/wp-content/uploads/2019/12/Met_rekom_1_god_.pdf
References
1. Ryumina I.I., Markelova M.M., Narogan M.V., Orlovskaya I.V., Perepelkina A.E., Ryndin A.Yu., Gatina E.A., Molkova E.A., Kosolapova Yu.A., Artamkina E.I., Sokolova E.V., Titova E.V., Kirillova E.A., Derevyagina O.S., Zubkov V.V., Baibarina E.N. Experience in implementing the International Standards for Assessing Newborn Growth INTERGROWTH-21st. Ros. Vestn. Perinatol. i Pediatr. 2021; 66:(1): 117–124 (In Russ.). DOI: 10.21508/1027-4065-2021-66-1-117-124
2. Andrews E.T., Beattie R.M., Johnson M.J. Measuring body composition in the preterm infant: Evidence base and practicalities. Clin. Nutr. 2019; 38(6): 2521–2530. DOI: 10.1016/j.clnu.2018.12.033
3. Kildiyarova R.R. Evaluation of physical development of newborns and children of early age. Ros. Vestn. Perinatol. i Pediatr. 2017; 62:(6): 62–68 (In Russ.). DOI: 10.21508/1027-4065-2017-626-62-68
4. Cormack B.E., Embleton N.D., van Goudoever J.B., Hay W.W. Jr, Bloomfi eld FH. Comparing apples with apples: it is time for standardized reporting of neonatal nutrition and growth studies. Pediatr. Res. 2016; 79(6): 810–820. DOI: 10.1038/pr.2016.26
5. Logutova L.S., Shilkina P.S. Modern aspects of the diagnosis and correction of feto-fetal transfusion syndrome in multifetal monochorial pregnancy. Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(3): 11–17 (In Russ.). DOI: 10.17116/rosakush20202003111
6. Markova T.V., Kosovtsova N.V., Chukanova A.N., Tsyv’ian P.B. Selective growth restriction in one of a monochorionic twin: Current management tactics. Russian Bulletin of Obstetrician-Gynecologist. 2016; 3: 42–47 (In Russ.). DOI: 10.17116/rosakush201616342-47
7. Belousova T.V., Andrushina I.V. Intrauterine growth retardation and its impact on children’s health in later life. the possibility of nutritional support. Current Pediatrics. 2015; 14(1): 23–30 (In Russ.). DOI: 10.15690/vsp.v14i1.1259
8. Shakaya M.N., Krogh-Jensen O.A., Ionov O.V. Newborn babies from multiple pregnancies complicated by twin-to-twin transfusion syndrome and selective intrauterine growth restriction syndrome. characteristics of the neonatal period. Neonatology: News, Opinions, Training. 2018; 6 (4(22)): 58–62 (In Russ). DOI: 10.24411/2308-2402-2018-14006
9. Tsibizova V.I., Govorov I.E., Averkin I.I., Khamani N.M., Blinov D.V. Health technology assessment in obstetrics: advantage of tailored conservative strategy vs surgical therapies of monochorionic twin complicated by TRAP-sequence. FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology. 2020; 13(1): 36–42 (In Russ.). DOI: 10.17749/20704909.2020.13.1.36-42
10. Kostyukov K.V., Sakalo V.A., Gladkova K.A., Shakaya M.N., Ionov O.V., Tetruashvili N.K. Perinatal outcomes of monochorionic multiple pregnancies complicated by twin-to-twin transfusion syndrome. Obstetrics and Gynecology. 2020; 8: 72–80 (In Russ.). DOI: 10.18565/aig.2020.8.72-80
11. Kosovtsova N.V., Putilova T.A., Pavlichenko M.V., Markova Т.V. Minimally invasive intrauterine interventions: A review of their use in the prevention of pregnancy complications in case of premature discharge of amniotic fl uid and rupture of the amniotic membrane during fetoscopic interventions. Journal of Obstetrics and Women’s Diseases. 2019; 68(4): 47–54 (In Russ.). DOI: 10.17816/JOWD68447-54
12. Van Mieghem T., Abbasi N., Shinar S., Keunen J., Seaward G., Windrim R., Ryan G. Monochorionic monoamniotic twin pregnancies. Am. J. Obstet. Gynecol. MFM. 2022; 4(2S): 100520. DOI: 10.1016/j.ajogmf.2021.100520
13. Sahno L.V., Bairova S.V., Koltunceva I.V., Gaiduk I.M., Revnova M.O., Mishkina T.V., et al. Current trends in the physical development of infants living in the northwest region. Pediatrician. 2019; 10(4): 17–24 (In Russ.). DOI: 10.17816/PED10417-24
14. Kalashnikov S.A. The course and outcomes of pregnancy in monochorionic twins. Russian Bulletin of Obstetrician-Gynecologist. 2021; 21(3): 85–91 (In Russ.). DOI: 10.17116/rosakush20212103185
15. Villar J., Cheikh Ismail L., Victora C.G., Ohuma E.O., Bertino E., Altman D.G., Lambert A., Papageorghiou A.T., Carvalho M., Jaffer Y.A., Gravett M.G., Purwar M., Frederick I.O., Noble A.J., Pang R., Barros F.C., Chumlea C., Bhutta Z.A., Kennedy S.H.; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014; 384(9946): 857–868. DOI: 10.1016/S01406736(14)60932-6
16. Villar J., Giuliani F., Fenton T.R., Ohuma E.O., Ismail L.C., Kennedy S.H.; INTERGROWTH-21st Consortium. INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016; 387(10021): 844– 845. DOI: 10.1016/S0140-6736(16)00384-6
17. Sun M., Lu J., Sun M., Zheng Y., Zhu Q., Liu C. Analysis of extrauterine growth retardation and related risk factors in 132 premature infants. Pak. J. Med. Sci. 2022; 38(6): 1644–1648. DOI: 10.12669/pjms.38.6.5864
18. Kholin A.M., Gus A.I., Khodzaeva Z.S., Baev O.R., Ryumina I.I., Villar J., Kennedy S., Papageorghiou A.T. Ways to standardise of fetometry in russia: INTERGROWTH-21st project and its implementation. Obstetrics and Gynecology. 2018; 9: 170–175 (In Russ.). DOI: 10.18565/aig.2018.9.170-175
19. Papageorghiou A.T., Kennedy S.H., Salomon L.J., Altman D.G., Ohuma E.O., Stones W., Gravett M.G., Barros F.C., Victora C., Purwar M., Jaffer Y., Noble J.A., Bertino E., Pang R., Cheikh Ismail L., Lambert A., Bhutta Z.A., Villar J.; International Fetal and Newborn Growth Consortium for the 21(st) Century (INTERGROWTH-21(st)). The INTERGROWTH21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am. J. Obste.t Gynecol. 2018; 218(2S): S630–S640. DOI: 10.1016/j.ajog.2018.01.011
20. Larsen M.L., Wiingreen R., Jensen A., Rackauskaite G., Laursen B., Hansen B.M., Hoei-Hansen C.E., Greisen G. The effect of gestational age on major neurodevelopmental disorders in preterm infants. Pediatr. Res. 2022; 91(7): 1906–1912. DOI: 10.1038/s41390-021-01710-4
21. Nemkova S.A. Modern principles of integrated diagnostics and rehabilitation of perinatal lesions of the nervous system and their consequences. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova. 2017; 117(3): 40–49 (In Russ.). DOI: 10.17116/jnevro20171173140-49
22. Pavlyukova E.V., Davydova I.V., Lazurenko S.B., Yatsyk G.V., Konova O.M., Zimina E.P. Opportunities for prevention and rehabilitation treatment of the consequences of perinatal central nervous system damage in premature infants. Pediatric Pharmacology. 2018; 15(2): 159–167 (In Russ.). DOI: 10.15690/pf.v15i2.1872
23. Weber A.M., Harrison T.M. Maternal behavior and infant physiology during feeding in premature and term infants over the fi rst year of life. Res. Nurs. Health. 2014; 37(6): 478–489. DOI: 10.1002/nur.21618
About the Authors
M. V. PavlichenkoRussian Federation
Maria V. Pavlichenko — Cand. Sci. (Med.), senior researcher, Head of the Early Childhood Department.
Repina str., 1, Ekaterinburg, 620028
N. V. Kosovtsova
Russian Federation
Natalya V. Kosovtsova — Dr. Sci. (Med.), Prof., doctor of the highest category; Head of the Department for Biophysical Research Methods.
Repina str., 1, Ekaterinburg, 620028
Ya. Yu. Pospelova
Russian Federation
Yana Yu. Pospelova — ultrasound specialist, graduate student.
Repina str., 1, Ekaterinburg, 620028
T. V. Markova
Russian Federation
Tatyana V. Markova — Cand. Sci. (Med.), senior researcher.
Repina str., 1, Ekaterinburg, 620028
Supplementary files
Review
For citations:
Pavlichenko M.V., Kosovtsova N.V., Pospelova Ya.Yu., Markova T.V. Physical development of preterm monochorionic diamniotic twins at birth: retrospective cohort study. Kuban Scientific Medical Bulletin. 2023;30(1):37-48. https://doi.org/10.25207/1608-6228-2023-30-1-37-48
JATS XML








































