Scroll to:
Complete blood count in healthy newborns in the first days of life (a comparative analysis against the 20th-century norms): A retrospective cohort study
https://doi.org/10.25207/1608-6228-2025-32-1-17-28
Abstract
Complete blood count is one of the most accessible and common laboratory tests available today. In present-day healthy newborns, complete blood count levels differ from the normative values used in the 1970s, which may require critical analysis, clarification, further research, and revision of the concept of norm.
Objectives. To identify changes in the hematologic parameters of healthy newborns born in 2022 as compared to those reported in the 1930s and 1967–1970s.
Methods. A retrospective cohort study of neonatal records was conducted at the Neonatal Physiology Unit of the Perinatal Center of the Saint Petersburg State Pediatric Medical University (Ministry of Health of the Russian Federation). Eleven hematologic parameters were analyzed in 378 healthy full-term newborns (born in 2022) in their first three days of life with subsequent comparison of the obtained results with the data of 20th-century domestic publications, as well as with Russian and foreign scientific articles published in the last 15 years. The blood of all children was tested once using a Micros ES 60 automated hematology analyzer (HORIBA ABX S.A.S., France). Mathematical and statistical data processing was performed using Microsoft Office Excel 2021 (Microsoft, U.S.).
Results. A comparison of red blood cell and hemoglobin levels reported in newborns in their second and third days of life in 1970 and 2022 revealed statistically significant differences, with lower levels observed at present. Mean platelet counts are also significantly lower than those reported in the 1930 and 1970 studies but are almost the same as those reported by other authors in 2012–2019. The mean white blood cell count is slightly higher as compared to the 20th-century normative values. The dynamics of changes in the mean white blood cell count are similar in all studies presented in the article, revealing a marked decrease by the third day of life as compared to the first and second days; however, 20th century data reveals even lower levels on the third day of life. Noteworthy is the decrease in the relative numbers of lymphocytes and band neutrophils with an increase in segmented neutrophils in this study as compared to the 1930, 1970, and 2012 studies.
Conclusion. The current mean values and reference intervals of hematologic parameters in healthy newborn babies differ from those presented in scientific studies and medical articles of the 20th century. This fact requires repeat analysis and establishment of new norms due to the increased sensitivity of analysis, the emergence of new procedures, and higher accuracy of equipment, as well as, potentially, due to changes in the maturity criteria and physical development indicators for newborns in the 21st century.
For citations:
Sakhno L.V., Koltuntceva I.V., Rasteryaev A.N., Bairova S.V., Shurygina K.B., Revnova M.O., Fedorova L.A., Levadneva M.I. Complete blood count in healthy newborns in the first days of life (a comparative analysis against the 20th-century norms): A retrospective cohort study. Kuban Scientific Medical Bulletin. 2025;32(1):17-28. https://doi.org/10.25207/1608-6228-2025-32-1-17-28
INTRODUCTION
Complete blood count (CBC) is included in the list of mandatory tests and is used by physicians in their everyday practice in the examination of healthy and sick children.
In the first days of life, the hematologic parameters of newborns constitute important indicators of their health and development. They reflect the condition of various organs and systems of the body, indicating the presence of pathological processes. Noteworthy is that recent decades have seen significant changes in the environmental situation, development and maturation of children, as well as considerable technological advances in laboratory diagnostics. The hemogram test has been used for over a century; the latest generations of analyzers are capable of performing about 100 analyses per hour, evaluating over 40 parameters and using 12–50 µL of biomaterial [1]. In the new reality, the use of outdated normative values for interpretive purposes is not well justified.
In 1930, on the basis of his observations, Alexander Tur determined and published norms for blood levels in healthy children, which immediately found wide application in pediatric practice. In over 35 years, children and their environment changed, which led to changes in hemogram parameters as well. In this connection, in 1966–1967, Alexander Tur and Nikolay Shabalov conducted a retrospective study examining a large number of healthy children from both Leningrad and other regions of the USSR. As a result, they published a monograph in 1970 for practitioners and scientific workers of various specialties, which not only revised some blood level norms established in 1930 but also compared their values with the data of other researchers.1
After 1970, domestic scientists continued to address the issue of pediatric blood level norms; however, these parameters have not been revised in the last 50 years.
In modern clinical guidelines and textbooks, authors refer to normative values that were established over 40–50 years ago. For example, the domestic Clinical Guidelines “Congenital Anemia due to Fetal Blood Loss” (2024)2 use the reference values of red blood cell levels that are derived from a 1977 study [2].
Few modern studies on blood level norms for children are available due to the ethical difficulty in finding volunteers, i.e., a sufficient number of healthy children of different ages and sexes. Foreign authors note that no normative CBC parameters are available for newborns because it is virtually impossible to obtain the approval of an ethical committee to collect blood samples for purely research purposes [3]. Blood drawing in newborns and infants is technically difficult due to the smaller size of anatomical structures and increased sensitivity to pain and medical procedures. Other pre-analytical factors that should be considered in determining reference intervals (RI) include food and medication intake, physical activity, body position, time of day, sample volume and type, presence of anticoagulants in the tubes, transport duration and temperature, method of storage and sample preparation for analysis, etc. [1].
A large number of aspects should be considered when establishing RIs: sex, age, ethnicity, geography, climate, seasonal changes, and dietary patterns. In pediatric practice, special attention is paid to the child’s age, the degree of organ development and its maturity [4]. The maturity criteria have changed in the 21st century (i.e., 37–42 weeks); in the 20th century, the child was considered full-term after 38 full weeks of gestation. In addition, a large number of studies focus on changes in the main indicators of physical development in children (body weight and length). New international standards have been created; their application in practical pediatrics is justified in the works of domestic authors [4–6]. We also assume that changes in the parameters may be related to the global shift in the age of first-time mothers in developed countries in the 21st century as compared to the 20th century (i.e., from 23–25 to 30–33 years).
When comparing known reference ranges, it is important to take possible genetic features of the population into account. Due to the multinationality and wide geographical area of Russia, such differences can be manifested at regional levels [4][7].
Thus, due to the specified factors, either outdated data and reference intervals or data borrowed from large foreign studies are often used in the literature (including educational literature and scientific works) and, therefore, the work of pediatricians, despite the greater accuracy and speed of research.
Unfortunately, most RIs for laboratory tests were established over four decades ago using analytical technologies that are largely outdated and cannot be considered relevant in terms of modern methods [1].
In a 2012 study, the authors note that normative white blood cell differential parameters presented in current neonatology guidelines often differ by several times.3
Given the difference in the limits of physiological variations in the CBC parameters of newborns, studies that provide a means to identify the possible causes of this variability for certain groups of children are of particular importance. In 2012, the Almazov Federal Heart, Blood, and Endocrinology Centre examined and statistically processed the hemogram test results of 298 “conditionally healthy” newborns (with unfavorable obstetric history; cephalohematoma; indications for conservative therapy of hyperbilirubinemia; clinical symptoms requiring differentiation between transient conditions and the developing pathological process). The relationship between changes in white blood cell levels at the beginning of the adaptation period and antenatal/intrapartum risk factors was proved. This dependence indicates the need for applying the established hemogram norms in clinical practice since the interpretation of examination results obtained for such newborns using available norms will be inaccurate.4
Of interest is the work of Nadezhda Eliseeva et al. published in 2019. [8], in which 574 newborns were studied. The authors believe that it is necessary to establish RIs for each type of analyzer and the hemogram parameters of children using a hospital database.
Foreign sources often consider the blood level norms in children in terms of various aspects: the region of residence, the sex of the child, and the type of analyzer used in the study. Large samples of full-term healthy newborns were used for CBC analysis in order to establish reference intervals [9], as well as to determine the features of hemograms in newborns of some countries or regions of a particular country [10][11]. Other observations were aimed at establishing norms for blood levels in children: hemoglobin, red blood cells, or platelets [3][9]. A prospective study was conducted to establish a hemogram RI for healthy newborns, with the blood analysis performed using a Sysmex XN-1000 automated hematology analyzer [12]. A large number of works are devoted to establishing RIs in children of different ages, including the hemogram parameters of children aged three days and older [13].
All of the above-mentioned issues may adversely affect the treatment and diagnostic process; the use of incorrect pediatric RIs will complicate the diagnostic search and may lead to repeated blood draws and increased duration of hospitalization.
The study aims to identify possible changes in the hematologic parameters of healthy newborns in 2022 as compared to parameters reported in the 1930s and 1967–1970s.
METHODS
Study design
The study design is based on a comparative analysis of data from a 2022 retrospective cohort study of normative hematologic parameters in conditionally healthy newborns (1–3 days of age) and data reported in 20th-century studies: 1930 and 1967–1970 studies by Academician Alexander Tur and Nikolay Shabalov, as well as data from 21st-century domestic authors5 [11].
Study conditions
The laboratory studies were conducted at the Neonatal Physiology Unit of the Perinatal Center at the Saint Petersburg State Pediatric Medical University (SPbSPMU). An analysis of the obtained results was performed at the Department of Pediatrics named after Academician A. F. Tur of SPbSPMU.
Eligibility criteria
Inclusion criteria
The study included healthy full-term (37–42 weeks of gestation) newborns with an Apgar score of 7–10 at birth. No pathologies were detected in the examined children at the time of their stay at the Perinatal Center; the children were discharged home on days 3–5 of life in a satisfactory condition; they did not receive antibacterial therapy at the maternity hospital. The CBC test was performed once due to risk factors for the mother (unfavorable obstetric history, etc.) or the child (clinical symptoms requiring differentiation between transient and pathologic conditions), and no repeat testing was required for clinical needs.
Exclusion criteria
Pre-term newborns; full-term newborns with an Apgar score of less than 7 at one minute of life and less than 9 at five minutes of life; discharge on day 6 or later; any primary or secondary diagnosis other than “healthy” at the discharge of a newborn.
Description of eligibility criteria (diagnostic criteria)
All full-term newborns who underwent CBC on one of the first days of life and were discharged from the Perinatal Center on days 3–5 of life with a diagnosis of “healthy”.
Selection of group members
The study group members (n = 378) were selected retrospectively using the clinical and medical history data of newborns and the results of the CBC test conducted on day 1 (n = 30), or day 2 (n = 301), or day 3 (n = 47) of life, taking the inclusion and exclusion criteria into account.
Target parameters in the study
Main parameter in the study
For all full-term newborns, the CBC was performed once.
Additional parameters in the study
Additional parameters are not provided in this study.
Methods for measuring the target parameters
Blood was drawn once from the newborns of the studied group to perform a CBC using a Micros ES 60 automated hematology analyzer (HORIBA ABX S.A.S., France), evaluating 11 hemogram parameters: red blood cell count (RBC, 10¹²/L); hemoglobin (Hgb, g/L); mean cell hemoglobin (MCH, pg); platelet count (PLT, 10⁹/L); white blood cell count (WBC, 10⁹/L); relative number of lymphocytes (LY, %), monocytes (MON, %), band and segmented neutrophils (GR, %), eosinophils (EOS, %), and basophils (BAS, %).
The obtained results were comparatively analyzed against the 20th-century normative values, which were found in literary sources.
Variables (predictors, confounders, and effect modifiers)
The confounding factors that can independently affect the hematologic parameters of CBC primarily include neonatal diseases. These factors were mitigated at the sampling stage through their addition to the exclusion criteria.
Statistical procedures
Principles behind sample size determination
The sample size was not determined in advance.
Statistical methods
The means (M) and standard deviations (SD) were calculated; the variation limits (min-max) for red and white blood cell levels in newborns were determined. Statistical data processing was performed using Microsoft Office Excel 2021 (Microsoft, U.S.) with an additional data analysis package. Data were described using the means (M) and standard deviations (SD). The Pearson’s χ² test without Yates’s correction was applied to analyze the distribution in the studied samples. The parametric Student’s t-test was used to compare independent groups in terms of quantitative characteristics. Differences were considered to be significant at p < 0.05.
RESULTS
Sampling
Children born at the Neonatal Physiology Unit of the Perinatal Center of SPbSPMU in 2022 served as the base population for the study. A total of 568 neonatal records of newborns who were discharged with a diagnosis of “healthy” and underwent at least one CBC were selected. Forty-five records with blood test data containing clerical errors or lost data were excluded. Five hundred and twenty-three records were screened according to the inclusion criteria. In the following cases, children (n = 145) were excluded from the study: gestational age of < 37 weeks; one or both Apgar scores were less than 7 at one minute of life or less than 9 at five minutes of life; biochemical blood analysis was performed and elevated bilirubin levels were noted; the CBC was performed more than once; discharge on day 6 or later. The block diagram of the study design is presented in the figure.
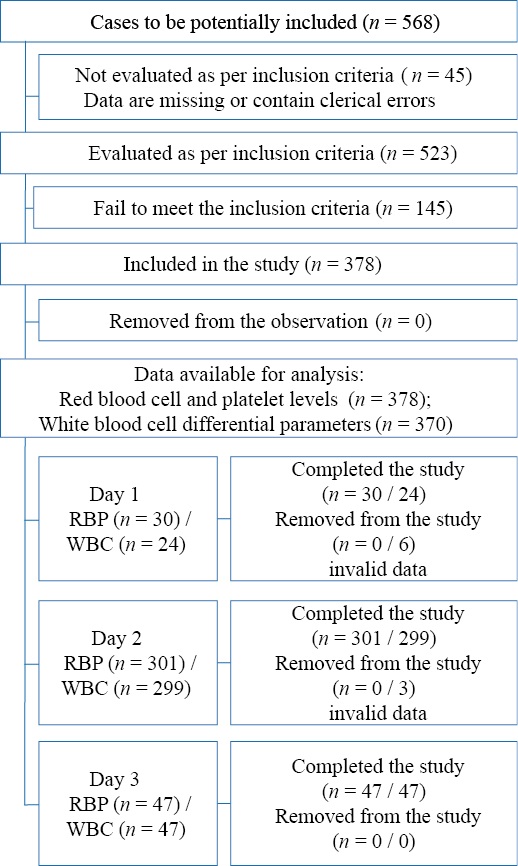
Figure. Block diagram of the study design
Note. The block diagram was created by the authors (as per STROBE recommendations). Abbreviations: RBP — red blood cell and platelet levels; WBC — white blood cell differential parameters.
Рисунок. Блок-схема дизайна исследования
Примечание: блок-схема выполнена авторами (согласно рекомендациям STROBE). Сокращения: RBP — показатели красной крови и тромбоцитов; WBC — показатели лейкоцитарной формулы.
Characteristics of the study sample (groups)
The study examined and analyzed the data of 378 children: 211 boys and 167 girls, with 164 children born naturally and 214 by cesarean section. The mean birth weight of the children was 3700 g and the mean body length was 53 cm. The mean age of maternity patients amounted to 33 years. Table 1 shows the medical data, body weight, and body length of the newborns divided into groups according to the day when the CBC was performed. No significant differences in the physical development indicators (body weight and length) were noted in the groups. In addition, the mean maternal age in all three groups also does not differ significantly (Table 2).
Table 1. Characteristics of the studied groups of newborns depending on the age at which the CBC was performed
Таблица 1. Характеристика групп исследования новорожденных в зависимости от возраста, в котором взят клинический анализ крови
|
Some parameters and medical data of newborns |
Day of life |
||
|
Day 1 (n = 30) |
Day 2 (n = 301) |
Day 3 (n = 47) |
|
|
Sex: |
|||
|
Male |
23 |
170 |
18 |
|
Female |
7 |
131 |
29 |
|
Body length*, m (M ± SD) |
0.52 ± 0.02 |
0.52 ± 0.06 |
0.53 ± 0.02 |
|
Body weight*, kg (M ± SD) |
3.39 ± 0.45 |
3.75 ± 0.47 |
3.56 ± 0.49 |
|
Maternal age*, years (M ± SD) |
32.77 ± 4.09 |
33.51 ± 5.02 |
32.02 ± 5.46 |
|
Physiologic childbirth |
11 (36.6%) |
127 (42.3%) |
26 (55.3%) |
|
Planned cesarean |
19 (63.3%) |
174 (57.7%;) |
21 (44.7%) |
Notes: The table was compiled by the authors; * no statistically significant differences in the parameter were found (р > 0.05).
Примечания: таблица составлена авторами; * статистически достоверных различий показателя не получено (р > 0,05).
The present authors did not aim to compare age-specific CBC parameters in children. In this study, the normative CBC values for newborns reported in the works of Alexander Tur (1930)6 and Nikolay Shabalov (1970),7 as well as domestic data reported in 2012,8 served as control groups.
Main study results
The mean CBC parameters in healthy newborns in 2022 were calculated and compared with the domestic normative values for newborns in the 20th century (1970).
The study and dynamics assessment of the mean red blood cell count in newborns in 2022 reveals a slight decrease in the levels in the first three days of life (5.79–5.38–5.17×10¹²/L); the total red blood cell count is lower than in the 19306 and 19707 studies (Table 2). No significant differences were found between this study and the 2012 study8 (Table 2).
Table 2. Red blood cell and platelet levels in healthy newborns in 1930, 1967–1970, 2012, and 2022.
Таблица 2. Показатели красной крови и тромбоцитов здоровых новорожденных 1930, 1967–1970, 2012, 2022 гг.
|
Parameters |
Day of life |
Alexander Tur (1930) |
Alexander Tur and Nikolay Shabalov (1967–1970) |
Elizaveta Kurzina et al. (2012) |
Present authors (2022) |
|
M* |
M ± SD |
M ± SD |
M ± SD |
||
|
Red blood cell count (10¹²/L) (RBC) |
1 |
6.62 |
6.09 ± 0.68* |
5.40 ± 0.75* |
5.79 ± 0.77 |
|
2 |
5.73 |
6.05 ± 0.66*** |
5.30 ± 0.60* |
5.38 ± 0.69 |
|
|
3 |
5.40 |
5.92 ± 0.68*** |
5.40 ± 0.60* |
5.17 ± 0.81 |
|
|
Hemoglobin (Hgb) |
1 |
124.00% 210.8 g/L |
212.0 ± 20.0* |
178.9 ± 21.8 |
202.33 ± 26.94 |
|
2 |
115.00% 195.5 g/L |
204.0 ± 19.0*** |
174.5 ± 18.25 |
188.86 ± 22.77 |
|
|
3 |
108.00% 183.6 g/L |
208.0 ± 22.0*** |
176.1 ± 20.7* |
180.79 ± 25.84 |
|
|
Mean cell hemoglobin (pg) (MCH) |
1 |
31.84 |
38.00 ± 4.00 |
33.13* |
34.95 ± 1.27 |
|
2 |
34.12 |
39.00 ± 3.00 |
32.92* |
35.25 ± 1.86 |
|
|
3 |
34.00 |
39.50 ± 3.00 |
32.61* |
35.14 ± 2.27 |
|
|
Platelet count (10⁹/L) (PLT) |
1 |
282.00 |
327.50 ± 110*** |
214.5 ± 75.2** |
241.43 ± 79.69 |
|
2 |
228.00 |
308.50 ± 114*** |
228.1 ± 79.55* |
250.94 ± 66.83 |
|
|
3 |
208.00 |
300.90 ± 107.5** |
235.3 ± 69.9* |
254.64 ± 74.05 |
Notes: The table was compiled by the authors; * no statistically significant differences in the parameter were found (р > 0.05); ** significance of the difference between the 2022 parameter and the author’s data of p < 0.005; *** significance of differences between the 2022 parameter and the author’s data of р < 0.0000001.
Примечания: таблица составлена авторами. * статистически достоверных различий показателя не получено (р > 0,05); ** достоверность различия показателя 2022 г. с данными автора р < 0,005; *** достоверность различий показателя 2022 г. с данными автора р < 0,0000001.
A comparison of the mean red blood cell counts in newborns shows a decrease in this parameter in 2022 as compared to the 19306 and 19707 studies (Table 2).
In a study by Alexander Tur (1930), the hemoglobin level was measured via the colorimetric method using a Migos nanometer, in which 100% hemoglobin corresponded to 17g% oxyhemoglobin. In 1967–1970, 100% corresponded to 16.7% hemoglobin7 in GS-2 hemometers; conversion to g/L was performed. Due to the use of different methods for statistical data processing in 1930 and 2022 and the lack of data on sample size and SD in the 1930 monograph by Alexander Tur (only mean, minimum, and maximum values of parameters are provided), it was not possible to determine the significance of differences between the obtained data and those reported in 1930. In this connection, the mean 1930 data are presented in tables for historical reference. In addition, the monograph of Alexander Tur and Nikolay Shabalov (1970) notes a change in red and white blood cell levels as compared to the previously published norms (1930), so it is logical to assume that if the 1970 and 2022 data are proved to be different, then the 1930 data are also different from 2022.
The dynamics assessment of hemoglobin levels in 2022 revealed a decrease from 202.33 to 180.79 g/L. The mean cell hemoglobin remained stable. Mean hemoglobin level and mean cell hemoglobin in children born in 2022 are slightly lower as compared to the parameters reported in 1930 and 1970 but higher than those in the study by Elizaveta Kurzina et al. (2012).
From day 1 to day 3 of life, a decrease in red blood cell parameters—hemoglobin and red blood cells—is observed (Table 2), with no significant changes in the mean cell hemoglobin. From day 1 to day 3, the mean platelet count remains almost the same.
A comparison of data on the red blood cell counts and hemoglobin levels on days 2 and 3 of life revealed statistically significant differences between the 1970 and 2022 data; on day 1 of life, these parameters do not differ significantly, which may be attributed to this group having the smallest number of newborns (n = 30). No significant differences in the number of red blood cells and hemoglobin levels were found between 2022 and 2012 data, which confirms our hypothesis about changes in some parameters due to the long period of time and biological changes in the population.
According to 2022 data, the mean platelet count does not change significantly in the first three days of life (241(250−254)×10⁹); however, a slight trend for higher platelet counts is observed, as in the study by Elizaveta Kurzina et al.9 Conversely, the 1930 and 1970 studies reported significantly higher platelet levels at birth (327 and 282×10⁹/L) and a significant downward trend (to 300 and 208×10⁹/L) (Table 2).
Noteworthy is that 2022 platelet counts remain almost stable throughout the first three days of life, thus confirming the stability of their production and destruction mechanism in the neonatal period. As compared to 1930 and 1970 data, a decrease in platelet levels at birth is observed; however, the upward trend throughout the first days of life remains. A comparison of platelet counts revealed statistically significant differences between the 2022 and 1970 data.
The 1970 data reveals a significantly lower total white blood cell count at birth (16.7×10⁹/L) as compared to all other studies where the values of this parameter are approximately the same ((23–27)×10⁹/L). The dynamics of changes reveal a marked decrease by day three of life compared to days one and two (Table 3). The same trend is observed in all compared studies; however, the 1930 and 1967–1970 data reveals lower levels on day 3 of life ((13.6–11.5)×10⁹/L) as compared to the 2012 and 2022 data ((17.1–16.86)×10⁹/L, respectively). The results of this study are comparable to the data obtained in the 2016 study analyzing the white blood cell counts of 476 healthy newborns, which were as follows: (22.5 ± 6.2)×10⁹/L at birth; (13.2 ± 3.7)×10⁹/L on day 2 of life; (12.3 ± 3.2)×10⁹/L on day 3 of life [14].
Table 3. White blood cell differential in healthy newborns in 1930, 1967–1970, 2012, and 2022.
Таблица 3. Показатели лейкоцитарной формулы здоровых новорожденных 1930, 1967 –1970, 2012, 2022 гг.
|
Parameters |
Day of life |
Alexander Tur (1930) |
Alexander Tur and Nikolay Shabalov (1967–1970) |
Elizaveta Kurzina et al. (2012) |
Present authors (2022) |
|
M* |
M ± SD |
M ± SD |
M ± SD |
||
|
White blood cell count (10⁹/L) (WBC) |
1 |
26.40 |
16.70 ± 5.34*** |
27.50 ± 8.20** |
23.12 ± 6.81 |
|
2 |
15.10 |
15.00 ± 4.90*** |
19.80 ± 6.00** |
22.75 ± 5.80 |
|
|
3 |
13.60 |
11.50 ± 3.74*** |
17.10 ± 6.80* |
16.86 ± 5.32 |
|
|
Band neutrophils (%) |
1 |
— |
6.60 ± 5.80** |
8.80 ± 6.85 |
3.88 ± 2.92 |
|
2 |
— |
5.90 ± 5.40** |
3.70 ± 3.40* |
3.64 ± 3.35 |
|
|
3 |
— |
3.80 ± 2.80** |
4.10 ± 3.50** |
2.00 ± 2.04 |
|
|
Segmented neutrophils (%) |
1 |
— |
61.20 ± 11.60* |
57.50 ± 8.85 |
64.75 ± 11.95 |
|
2 |
— |
58.00 ± 11.10*** |
57.50 ± 11.35* |
63.32 ± 8.13 |
|
|
3 |
— |
52.50 ± 11.00*** |
51.40 ± 12.40 |
60.17 ± 7.03 |
|
|
Eosinophils (%) |
1 |
2.16 |
2.60 ± 1.90* |
1.40 ± 1.70* |
1.92 ± 2.41 |
|
2 |
3.00 |
2.90 ± 2.10** |
2.20 ± 1.02* |
2.09 ± 2.04 |
|
|
3 |
3.00 |
3.70 ± 2.00* |
3.00 ± 2.50* |
3.28 ± 2.29 |
|
|
Basophils (%) |
1 |
0.50 |
— |
0.30 ± 0.60 |
0.33 ± 0.99 |
|
2 |
0.00 |
— |
0.30 ± 0.65 |
0.66 ± 1.36 |
|
|
3 |
0.00 |
— |
0.20 ± 0.50 |
0.57 ± 1.40 |
|
|
Lymphocytes (%) |
1 |
22.16 |
23.60 ± 8.10* |
24.40 ± 7.78 |
21.38 ± 10.99 |
|
2 |
24.00 |
26.70 ± 8.10** |
29.40 ± 10.95 |
22.19 ± 7.72 |
|
|
3 |
30.50 |
31.10 ± 9.20* |
33.00 ± 11.30 |
26.09 ± 6.92 |
|
|
Monocytes (%) |
1 |
9.00 |
7.30 ± 3.20* |
6.30 ± 2.55 |
7.67 ± 2.69 |
|
2 |
10.50 |
8.40 ± 3.70* |
6.50 ± 2.70 |
7.96 ± 2.85 |
|
|
3 |
11.00 |
10.10 ± 4.20* |
7.90 ± 3.20* |
8.00 ± 2.95 |
Notes: The table was compiled by the authors; * no statistically significant differences between the 2022 parameter and the author’s data were found (р > 0.05); ** significance of the difference between the 2022 parameter and the author’s data of р < 0.01; *** significance of differences between the 2022 parameter and the author’s data of p < 0.0000001.
Примечания: таблица составлена авторами; * статистически достоверных различий показателя 2022 г. с данными автора не получено (р > 0,05); ** достоверность различия показателя 2022 г с данными автора р < 0,01; *** достоверность различий показателя 2022 г с данными автора р < 0,0000001.
The relative number of segmented neutrophils (1970–2022) also exhibits a downward trend; however, it is higher by 3–6% on days 1 and 2 of life and by 7–8% on day 3 in the present study as compared to the 1970 and 2012 data (Table 3). In this study, the number of band neutrophils in children on day 1 of life is significantly lower than in the 1970 and 2012 studies (3.88, 6.60, and 8.80%, respectively). The downward trend on day 3 is slight and similar to that in 1970 (3.88–2.00 and 6.60–4.10%, respectively). Significantly different are the 2012 data that showed high levels at birth with a decline by day 2 and a slight increase by day 3 (8.8, 3.7, and 4.1%, respectively). The parameters measured on day 2 of life are similar to the 2022 data (Table 3). A comparison of 1970 and 2022 parameters revealed significant differences in the relative number of band neutrophils on days 1–3 of life and segmented neutrophils on days 2–3 of life in newborns.
From day 1 to day 3 of life, the relative number of lymphocytes increases; noteworthy is that the variation in the levels reported in all studies (1930, 1967, 2012, and 2022) is not very significant (Table 3), while the relative number of lymphocytes is significantly different on day 2 of life in 1970 and 2022.
A comparison of the relative numbers of basophils and monocytes (Table 3) revealed no significant differences between the 1930, 1967–1970, 2012, and 2022 studies in the first three days of the child’s life, while the relative number of eosinophils differed only on day 2 in 1970 and 2022.
Additional study results
No additional results were obtained during the study.
DISCUSSION
Summary of the main study result
Thus, the levels of red blood cells, platelets, and white blood cells changed in 1930–2022, which may be attributed to changes in diagnostic methods, as well as evolutionary and socioecological factors. A decrease in the relative number of segmented neutrophils with the increasing number of lymphocytes reflects normal physiological processes of adaptation in newborns. These changes should be taken into account when assessing a child’s health status to avoid overdiagnosis of pathologic conditions. In performing clinical tasks and deciding whether to classify a patient as healthy or sick, the pediatrician relies on clinical decision limits, which are determined by the reference intervals of parameters.
Research limitation
Given the differences in hematologic parameters of healthy newborns in the first three days of life in 2022 as compared to children born in the 1930s and 1960s, we can also assume changes in the blood level norms for healthy children from other age groups. This can be attributed to new procedures and equipment, as well as to the features of the region where the subjects live.
Interpretation of the study results
A large number of works currently focus on indirect methods of RI estimation. The developed computational algorithms [15–17] for establishing norms reflect the biological properties of the population used to estimate the RI and constitute a model of non-pathological distribution (an alternative to direct methods requiring samples from healthy individuals).
Noteworthy is that the obtained levels of red blood cells, white blood cells, and platelets in newborns on day 1 of life are comparable to data from a multicenter prospective cohort study that examined 3325 healthy full-term children in 2019–2020 [18].
The difference in hemogram parameters of newborns in the 21st and 20th centuries can be attributed to the difference in the used methods (automated analyzers at present; microscope and manual counting in the 20th century), as well as to changes in the maturity criterion (37–42 weeks at present; 38–42 weeks in the 20th century), changes in the maternal age (shift in the age of first-time mothers to 30 years as compared to that in the 20th century), and changes in the physical data of newborns [5][6]. Therefore, we find the traditional method for evaluating big data with the exclusion of pathologies in age-specific groups of children to be more accurate than indirect RI estimation methods.
A comparative analysis of the present-day hemogram data of healthy newborns in the first three days of life (total red blood cell, platelet, and white blood cell counts; relative number of neutrophils, segmented and band neutrophils, lymphocytes, monocytes, basophils, eosinophils; hematocrit, and hemoglobin) against the normative values of the 20th century, as well as a study of domestic10 [8][15][17] and foreign [19–21] literature sources, reveals the need to revise the normative values of mean parameters and reference intervals of complete blood count.
Due to the large amounts of data stored on the servers of various medical institutions, it is now possible to conduct large-scale studies to specify or revise the reference intervals of age-specific blood level norms for children of different ages.
A correct CBC interpretation provides physicians with valuable data, which often allow even minor deviations from the norm to be detected in the child’s condition when other objective clinical methods reveal nothing about the dysfunction of certain organs [5][6][8].
Without a doubt, the normative values of laboratory parameters depend on a large number of aspects of the individual’s health and, above all, on clinical symptomatology, requiring a personalized approach in pediatric practice. The establishment of new hemogram norms for newborns and children of different ages on the basis of large medical databases will allow a practicing physician to promptly determine whether in-depth diagnostics is necessary in cases where the results deviate from the RI, even in the context of overall health [1][4][12].
The conducted study revealed the high significance of specifying reliable pediatric reference intervals and the need for a more detailed and large-scale approach to studying the problem under current conditions.
CONCLUSION
Current mean values and reference intervals of hematologic parameters in healthy newborns differ from parameters presented in scientific studies and medical articles of the 20th century. This fact requires repeat analysis and establishment of new norms due to the increased sensitivity of analysis, the emergence of new procedures, and higher accuracy of equipment, as well as, potentially, due to changes in the maturity criteria and physical development indicators for newborns in the 21st century. The health care system in Russia is undergoing digitalization, and a large amount of data is currently available on the servers of various medical institutions (municipal, regional, and private entities). Therefore, it seems feasible nowadays to conduct large-scale studies to adjust or update age-specific physiological blood level norms for children of different ages, thus justifying clinical decision limits in current pediatric practice.
1. Tur A.F., Shabalov N.P. Blood levels in healthy children of different ages. USSR Academy of Medical Sciences, Moscow: Meditsina, 1970. (In Russ.)
2. All-Russian Non-governmental Neonatology Development Organization “Russian Neonatology Society” (RNS); Association of Perinatal Medicine, Prenatal Care, and Provision of Care to Newborns and Children up to Three Years of Age (ASPM+). Congenital Anemia due to Fetal Blood Loss and Other Congenital Anemias in Newborns. Clinical Guidelines. 2024. (In Russ.) Available: https://opcyar.ru/docs/standarts/Неонатология%20Клинические%20рекомендации/Врожденная%20анемия%20вследствие%20кровопотери%20у%20плода.pdf
3. Kurzina E.A., Petrenko Yu.V., Ivanov D.O. Complete blood count in newborns during adaptation. Bulletin of Almazov Federal Heart, Blood and Endocrinology Centre. 2012;1:56–60. (In Russ.)
4. All-Russian Non-governmental Neonatology Development Organization “Russian Neonatology Society” (RNS); Association of Perinatal Medicine, Prenatal Care, and Provision of Care to Newborns and Children up to Three Years of Age (ASPM+). Congenital Anemia due to Fetal Blood Loss and Other Congenital Anemias in Newborns. Clinical Guidelines. 2024. (In Russ.) Available: https://opcyar.ru/docs/standarts/Неонатология%20Клинические%20рекомендации/Врожденная%20анемия%20вследствие%20кровопотери%20у%20плода.pdf
5. Tur A.F. Practical pediatric hematology. Moscow; Leningrad: Gos. Med. Izd-vo, 1931. (In Russ.)
Tur A.F., Shabalov N.P. Blood levels in healthy children of different ages. USSR Academy of Medical Sciences, Moscow: Meditsina, 1970. (In Russ.)
6. Tur A.F. Practical pediatric hematology. Moscow; Leningrad: Gos. Med. Izd-vo, 1931. (In Russ.)
7. Tur A.F., Shabalov N.P. Blood levels in healthy children of different ages. USSR Academy of Medical Sciences, Moscow: Meditsina, 1970. (In Russ.)
8. Kurzina E.A., Petrenko Yu.V., Ivanov D.O. Complete blood count in newborns during adaptation. Bulletin of Almazov Federal Heart, Blood and Endocrinology Centre. 2012;1:56–60. (In Russ.)
9. Kurzina E.A., Petrenko Yu.V., Ivanov D.O. Complete blood count in newborns during adaptation. Bulletin of Almazov Federal Heart, Blood and Endocrinology Centre. 2012;1:56–60. (In Russ.)
10. All-Russian Non-governmental Neonatology Development Organization “Russian Neonatology Society” (RNS); Association of Perinatal Medicine, Prenatal Care, and Provision of Care to Newborns and Children up to Three Years of Age (ASPM+). Congenital Anemia due to Fetal Blood Loss and Other Congenital Anemias in Newborns. Clinical Guidelines. 2024. (In Russ.) Available: https://opcyar.ru/docs/standarts/Неонатология%20Клинические%20рекомендации/Врожденная%20анемия%20вследствие%20кровопотери%20у%20плода.pdf
Kildiyarova R.R. Laboratornye i funktsional’nye issledovaniya v praktike pediatra [Laboratory and functional tests in pediatric practice]. 5th ed., rev. and suppl. Moscow: GEOTAR-Media, 2022. 192 p. (In Russ.)
References
1. Shamratova AR, Shamratova VG, Kayumovа AF, Ziyakaeva KR. The Capabilities of Haematology Analysers for Assessing the Bodyʼs Physiological and Pathological Conditions (Review). Journal of Medical and Biological Research. 2021;9(1):89–101 (In Russ.). https://doi.org/10.37482/2687-1491-Z047
2. Balashova EN, Sharafutdinova DR, Narogan MV, Sapun OI, Karpova AL, Senkevich OA, Kirtbay a АR, Ryndin AYu, Golubtsova YuM, Ionov OV, Zubkov VV, Degtyarev DN. Congenital anemia due to fetal blood loss (guideline). Neonatology: News, Opinions, Training. 2021;9(4):58–68 (In Russ.). https://doi.org/10.33029/2308-2402-2021-9-4-58-68
3. Cui D, Hou Y, Feng L, Li G, Zhang C, Huang Y, Fan J, Hu Q. Capillary blood reference intervals for platelet parameters in healthy full-term neonates in China. BMC Pediatr. 2020;20(1):471. https://doi.org/10.1186/s12887-020-02373-6
4. Chernova G.V., Sidorov P.V., Timofeeva M.A., Ergolskaya N.V., Sidorov V.V., Shiryaeva L.V. Osobennosti proyavleniya gematologicheskix priznakov v ix sopryazhennom raznoobrazii s pokazatelyami fizicheskogo razvitiya u detej perioda novorozhdennosti [Features of the manifestation of hematological signs in their associated diversity with indicators of physical development in children of the neonatal period]. Novy`e issledovaniya. 2020;1(61):57–68 (In Russ.).
5. Alyamovskayа GA, Sakharova ЕS, Keshishyan ES. Modern approaches to the physical development indicators in children in their first months of life. Russian Bulletin of Perinatology and Pediatrics. 2020;65(2):15–21 (In Russ.). https://doi.org/10.21508/1027-4065-2020-65-2-15-21
6. Ryumina II, Baibarina EN, Narogan MV, Markelova MM, Orlovskaya IV, Zubkov VV, Degtyarev DN. The usage of the international growth standards to assess the physical development of newborn and premature children. Neonatology: News, Opinions, Training. 2023;11(2):48–52 (In Russ.). https://doi.org/10.33029/2308-2402-2023-11-2-48-52
7. Chernova G, Sidorov VV, Shiryaeva LV, Timofeeva MA, Petrosyan VV. Variability of the erythroid indices in peripheral blood in children of the first year of life as a manifestation of genotype ecological effects. Public Health and Life Environment — PH&LE. 2019;2:26–31 (In Russ.).
8. Eliseeva NA, Saveliev LI, Philinkova EA, Tsvirenko SV. Reference intervals for complete blood count parameters in newborns during first day of life for the analyzer Sysmex XN-1000. Laboratory Service. 2019;8(3):31–36 (In Russ.). https://doi.org/10.17116/labs2019803131
9. Henry E, Christensen RD. Reference Intervals in Neonatal Hematology. Clin Perinatol. 2015;42(3):483–497. https://doi.org/10.1016/j.clp.2015.04.005
10. Price MA, Fast PE, Mshai M, Lambrick M, Machira YW, Gieber L, Chetty P, Muturi-Kioi V. Region-specific laboratory reference intervals are important: A systematic review of the data from Africa. PLOS Glob Public Health. 2022;2(11):e0000783. https://doi.org/10.1371/journal.pgph.0000783
11. Tiruneh T, Kiros T, Getu S. Hematological reference intervals among full-term newborns in Ethiopia: a cross-sectional study. BMC Pediatr. 2020;20(1):417. https://doi.org/10.1186/s12887-020-02320-5
12. Ianni B, McDaniel H, Savilo E, Wade C, Micetic B, Johnson S, Gerkin R. Defining Normal Healthy Term Newborn Automated Hematologic Reference Intervals at 24 Hours of Life. Arch Pathol Lab Med. 2021;145(1):66–74. https://doi.org/10.5858/arpa.2019-0444-OA
13. Mohammadi M, Ghazizadeh H, Mohammadi-Bajgiran M, Kathryn Bohn M, Yaghooti-Khorasani M, Kamel Khodabandeh A, Steele S, Torabzadeh Khorasani N, Ferns GA, Boskabadi H, Esmaily H, Adeli K, Assaran Darban R, Ghayour-Mobarhan M. Pediatric reference intervals for hematology parameters in healthy infants and young children in Iran. Int J Lab Hematol. 2023;45(6):845–852. https://doi.org/10.1111/ijlh.14132
14. Baranovskaya IB, Samokhina OF, Sysoeva IP. Pokazateli lejkocitarnogo analiza novorozhdenny`x pervy`x dnej zhizni [Indicators of leukocyte analysis of newborns in the first days of life]. Poliklinika. 2016;6:34–38 (In Russ.).
15. Plekhanova OS, Tsvirenko SV, Kalacheva OS, Saveliev LI. Evaluation of various indirect methods for calculating the reference intervals of the main CBC parameters in newborns 0–3 days of life. Laboratory Service. 2023;12(2):33–43 (In Russ.). https://doi.org/10.17116/labs20231202133
16. Ammer T, Schützenmeister A, Prokosch HU, Rauh M, Rank CM, Zierk J. refineR: A Novel Algorithm for Reference Interval Estimation from Real-World Data. Sci Rep. 2021;11(1):16023. https://doi.org/10.1038/s41598-021-95301-2
17. Evgina SA, Saveliev LI. Current theory and practice of reference interval36. Laboratory Service. 2019;8(2):36–44 (In Russ.). https://doi.org/10.17116/labs2019802136
18. Karpova AL. Complete blood count: reference intervals for full-term and late premature infants in the first day of life (part I). Pediatria Journal named after GN Speransky. 2022;101(1):62–70. http://dx.doi.org/10.24110/0031-403x-2022-101-1-62-70
19. Mohammadi M, Ghazizadeh H, Mohammadi-Bajgiran M, Kathryn Bohn M, Yaghooti-Khorasani M, Kamel Khodabandeh A, Steele S, Torabzadeh Khorasani N, Ferns GA, Boskabadi H, Esmaily H, Adeli K, Assaran Darban R, Ghayour-Mobarhan M. Pediatric reference intervals for hematology parameters in healthy infants and young children in Iran. Int J Lab Hematol. 2023;45(6):845–852. https://doi.org/10.1111/ijlh.14132
20. Bahr TM, Christensen TR, Henry E, Wilkes J, Ohls RK, Bennett ST, Ward DM, Pysher TJ, Christensen RD. Neonatal Reference Intervals for the Complete Blood Count Parameters MicroR and HYPO-He: Sensitivity Beyond the Red Cell Indices for Identifying Microcytic and Hypochromic Disorders. J Pediatr. 2021;239:95–100.e2. https://doi.org/10.1016/j.jpeds.2021.08.002 https://doi.org/10.1093/ajcp/aqaa059
21. Tahmasebi H, Higgins V, Bohn MK, Hall A, Adeli K. CALIPER Hematology Reference Standards (I). Am J Clin Pathol. 2020;154(3):330–341.
About the Authors
L. V. SakhnoRussian Federation
Larisa V. Sakhno — Cand. Sci. (Med.), Assoc. Prof., Department of Pediatrics named after Academician A. F. Tur
Litovskaya str., 2, St. Petersburg, 194100
I. V. Koltuntceva
Inna V. Koltuntceva — Cand. Sci. (Med.), Assoc. Prof., Department of Pediatrics named after Academician A. F. Tur
Litovskaya str., 2, St. Petersburg, 194100
A. N. Rasteryaev
Anatoly N. Rasteryaev — Department Assistant, Department of Pediatrics named after Academician A. F. Tur
Litovskaya str., 2, St. Petersburg, 194100
S. V. Bairova
Svetlana V. Bairovа — Cand. Sci. (Med.), Assoc. Prof., Department of Pediatrics named after Academician A. F. Tur
Litovskaya str., 2, St. Petersburg, 194100
K. B. Shurygina
Kseniya B. Shurygina — Department Assistant, Department of Pediatrics named after Academician A. F. Tur
Litovskaya str., 2, St. Petersburg, 194100
M. O. Revnova
Maria O. Revnova — Dr. Sci. (Med.), Prof., Departmental Head, Department of Pediatrics named after Academician A. F. Tur
Litovskaya str., 2, St. Petersburg, 194100
L. A. Fedorova
Larisa A. Fedorova — Cand. Sci. (Med.), Assoc. Prof., Neonatology Department with Courses in Neurology and Obstetrics & Gynecology, Faculty of Postgraduate and Additional Professional Development
Litovskaya str., 2, St. Petersburg, 194100
M. I. Levadneva
Marina I. Levadneva — Head of the Neonatal Physiology Unit; Teaching Assistant, Neonatology Department with Courses in Neurology and Obstetrics & Gynecology, Faculty of Postgraduate and Additional Professional Development
Litovskaya str., 2, St. Petersburg, 194100
Supplementary files
Review
For citations:
Sakhno L.V., Koltuntceva I.V., Rasteryaev A.N., Bairova S.V., Shurygina K.B., Revnova M.O., Fedorova L.A., Levadneva M.I. Complete blood count in healthy newborns in the first days of life (a comparative analysis against the 20th-century norms): A retrospective cohort study. Kuban Scientific Medical Bulletin. 2025;32(1):17-28. https://doi.org/10.25207/1608-6228-2025-32-1-17-28








































